Pediatr Infect Vaccine.
2016 Aug;23(2):94-101. 10.14776/piv.2016.23.2.94.
A Tuberculosis Contact Investigation on Health Care Workers in One Hospital
- Affiliations
-
- 1Department of Pediatrics, Hanyang University Hospital, Seoul, Korea. sungheeo@hanyang.ac.kr
- 2Department of Infection Control, Hanyang University Hospital, Seoul, Korea.
- 3Department of Internal Medicine, National Medical Center, Seoul, Korea.
- 4Department of Internal Medicine, Hanyang University Hospital, Seoul, Korea.
- KMID: 2353389
- DOI: http://doi.org/10.14776/piv.2016.23.2.94
Abstract
- PURPOSE
This study aimed to describe the results of a contact investigation on health care workers after exposure to a house officer with smear-positive pulmonary tuberculosis (TB).
METHODS
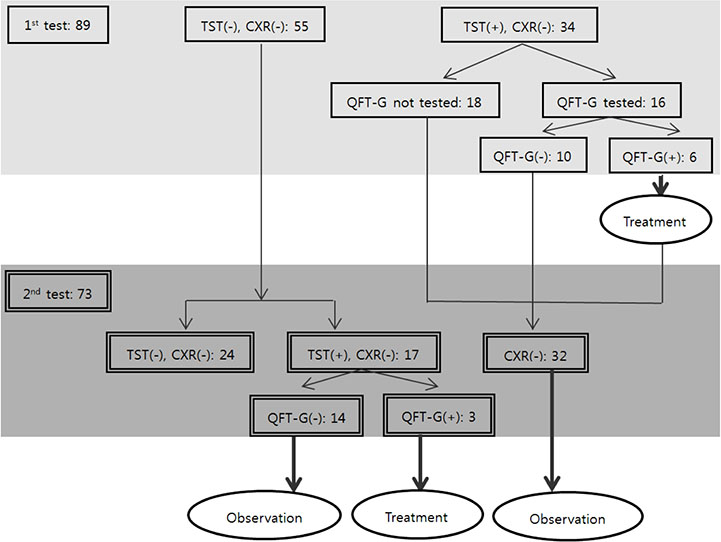
Eighty nine out of 101 subjects who had close contact with the index patient agreed to be enrolled in the investigation. The first contact investigation was conducted approximately 30 days after the index patient's onset of symptoms, followed by the second investigation after 10 weeks. In both, clinical manifestations were studied, and chest X-ray and tuberculin skin test (TST)/QuantiFERON-TB Gold (QFT-G) in dual screening strategy were conducted.
RESULTS
The first TST resulted in positive in 34 subjects (38.2%). QFT-G was conducted on 16 subjects who tested positive in the first TST and aged under 36. Six of them (37.5%) were positive. The second TST was conducted on 41 subjects with negative results in the first TST. Seventeen (41.5%) were positive and among them, three (17.6%) showed positive QFT-G. None of the subjects were diagnosed with active TB. The probability of TB infection through contact with the index patient was 7.3% (3/41) in dual screening strategy while it was 41.5% (17/41) in TST strategy.
CONCLUSIONS
This first hospital-setting contact investigation for tuberculosis in Korea revealed that latent tuberculosis infection (LTBI) rates vary depending on different diagnostic strategies. This indicates the need for systematic guidelines for diagnosing LTBI in health care workers who have professional exposure to TB.
Keyword
MeSH Terms
Figure
Reference
-
1. World Health Organization. Global tuberculosis report 2014. Geneva: World Health Organization;2014.2. Yang SH, Chu MA, Park HJ, Lee KH, Kim JK, Choi EJ, et al. Clinical characteristics of tuberculosis in school-age children and adolescents at a single institution. Pediatr Allergy Respir Dis. 2012; 22:239–247.
Article3. Styblo K. Recent advances in epidemiological research in tuberculosis. Adv Tuberc Res. 1980; 20:1–63.4. Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999; 282:677–686.5. Shim TS, Koh WJ, Yim JJ, Lew WJ. Treatment of latent tuberculosis infection in Korea. Tuberc Respir Dis. 2008; 65:79–90.
Article6. Chen TC, Lu PL, Yang CJ, Lin WR, Lin CY, Jou R, et al. Management of a nosocomial outbreak of Mycobacterium tuberculosis Beijing/W genotype in Taiwan: an emphasis on case tracing with high-resolution computed tomography. Jpn J Infect Dis. 2010; 63:199–203.
Article7. Jonsson J, Kan B, Berggren I, Bruchfeld J. Extensive nosocomial transmission of tuberculosis in a low-incidence country. J Hosp Infect. 2013; 83:321–326.
Article8. Centers for Disease Control and Prevention. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005; 54(RR-17):1–141.9. Korea Centers for Disease Control and Prevention. National guidelines for control and prevention of tuberculosis. Osong: Korea Centers for Disease Control and Prevention;2013.10. Oxlade O, Schwartzman K, Menzies D. Interferon-gamma release assays and TB screening in high-income countries: a cost-effectiveness analysis. Int J Tuberc Lung Dis. 2007; 11:16–26.11. Wrighton-Smith P, Zellweger JP. Direct costs of three models for the screening of latent tuberculosis infection. Eur Respir J. 2006; 28:45–50.
Article12. Joint Committee for the Revision of Korean Guidelines for Tuberculosis, Korean Centers for Disease Control and Prevention. Korean guidelines for tuberculosis. 2nd ed. Seoul and Cheongwon: Joint Committee for the Revision of Korean Guidelines for Tuberculosis, Korea Centers for Disease Control and Prevention;2014.13. Horsburgh CR Jr. Tuberculosis. Eur Respir Rev. 2014; 23:36–39.
Article14. Tuberculosis Research Centre, Indian Council of Medical Research, Chennai, India. Risk of tuberculosis among contacts of isoniazid-resistant and isoniazid-susceptible cases. Int J Tuberc Lung Dis. 2011; 15:782–788.15. Jo KW, Woo JH, Hong Y, Choi CM, Oh YM, Lee SD, et al. Incidence of tuberculosis among health care workers at a private university hospital in South Korea. Int J Tuberc Lung Dis. 2008; 12:436–440.16. Riva MA, Ploia PR, Rocca S, Cesana G. "Phthisiophobia": the difficult recognition of transmission of tuberculosis to health care workers. Med Lav. 2013; 104:359–367.17. Pai M, Denkinger CM, Kik SV, Rangaka MX, Zwerling A, Oxlade O, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev. 2014; 27:3–20.
Article18. Landry J, Menzies D. Preventive chemotherapy. Where has it got us? Where to go next? Int J Tuberc Lung Dis. 2008; 12:1352–1364.19. Farhat M, Greenaway C, Pai M, Menzies D. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis. 2006; 10:1192–1204.20. Bugiani M, Borraccino A, Migliore E, Carosso A, Piccioni P, Cavallero M, et al. Tuberculin reactivity in adult BCG-vaccinated subjects: a cross-sectional study. Int J Tuberc Lung Dis. 2003; 7:320–326.21. Mauch H, Brehmer W. Mycobacterial antibodies after tuberculin testing, BCG-vaccination, BCG-immunotherapy and against cross-reacting antigens in a solid-phase radioimmunoassay. Zentralbl Bakteriol Mikrobiol Hyg A. 1982; 251:380–388.
Article22. Zwerling A, van den Hof S, Scholten J, Cobelens F, Menzies D, Pai M. Interferon-gamma release assays for tuberculosis screening of healthcare workers: a systematic review. Thorax. 2012; 67:62–70.
Article23. Ringshausen FC, Schablon A, Nienhaus A. Interferon-gamma release assays for the tuberculosis serial testing of health care workers: a systematic review. J Occup Med Toxicol. 2012; 7:6.
Article24. Fong KS, Tomford JW, Teixeira L, Fraser TG, van Duin D, Yen-Lieberman B, et al. Challenges of interferon-gamma release assay conversions in serial testing of health-care workers in a TB control program. Chest. 2012; 142:55–62.
Article25. Dorman SE, Belknap R, Graviss EA, Reves R, Schluger N, Weinfurter P, et al. Interferon-gamma release assays and tuberculin skin testing for diagnosis of latent tuberculosis infection in healthcare workers in the United States. Tuberculosis Epidemiologic Studies Consortium. Am J Respir Crit Care Med. 2014; 189:77–87.26. Centers for Disease Control and Prevention. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010; 59:1–25.27. National Institute for Health and Clinical Excellence (NICE). Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. Royal College of Physicians of London;2011.28. Van Zyl-Smit RN, Zwerling A, Dheda K, Pai M. Within-subject variability of interferon-g assay results for tuberculosis and boosting effect of tuberculin skin testing: a systematic review. PLoS One. 2009; 4:e8517.
Article29. Diel R, Goletti D, Ferrara G, Bothamley G, Cirillo D, Kampmann B, et al. Interferon-gamma release assays for the diagnosis of latent Mycobacterium tuberculosis infection: a systematic review and meta-analysis. Eur Respir J. 2011; 37:88–99.
Article30. Mulder C, van Deutekom H, Huisman EM, Toumanian S, Koster BF, Meijer-Veldman W, et al. Role of the QuantiFERON (R)-TB Gold In-Tube assay in screening new immigrants for tuberculosis infection. Eur Respir J. 2012; 40:1443.
Article31. Menzies R, Vissandjee B, Rocher I, St Germain Y. The booster effect in two-step tuberculin testing among young adults in Montreal. Ann Intern Med. 1994; 120:190–198.
Article32. Lee SW, Oh DK, Lee SH, Kang HY, Lee CT, Yim JJ. Time interval to conversion of interferon-gamma release assay after exposure to tuberculosis. Eur Respir J. 2011; 37:1447–1452.
Article33. Balmelli C, Zysset F, Pagnamenta A, Franciola P, Lazor-Blanchet C, Zanetti G, et al. Contact tracing investigation after professional exposure to tuberculosis in a Swiss hospital using both tuberculin skin test and IGRA. Swiss Med Wkly. 2014; 144:w13988.
Article34. Li CY, Chen HC, Cheng HY, Chian CF, Chang FY, Chen HI, et al. Role of QuantiFERON-TB-Gold In Tube assay for active and latent tuberculosis infection in investigation of tuberculosis outbreak in a university. J Microbiol Immunol Infect. 2015; 48:263–268.
Article35. O'Neal S, Hedberg K, Markum A, Schafer S. Discordant tuberculin skin and interferon-gamma tests during contact investigations: a dilemma for tuberculosis controllers. Int J Tuberc Lung Dis. 2009; 13:662–664.36. Nienhaus A, Schablon A, Diel R. Interferon-gamma release assay for the diagnosis of latent TB infection--analysis of discordant results, when compared to the tuberculin skin test. PLoS One. 2008; 3:e2665.37. Lee SS, Liu YC, Huang TS, Chen YS, Tsai HC, Wann SR, et al. Comparison of the interferon-gamma release assay and the tuberculin skin test for contact investigation of tuberculosis in BCG-vaccinated health care workers. Scand J Infect Dis. 2008; 40:373–380.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Occupational Dermatoses of Health Care Workers in Korea
- Contact Investigation for Twins With Congenital Tuberculosis in the Neonatal Intensive Care Unit
- Diagnosis and Treatment of Latent Tuberculosis Infection in Healthcare Workers
- Personnel Management of Tuberculosis Workers: A Survey on the Job, Training and Performance of the Field Workers in Korea
- Prevention of tuberculosis and isolation of tuberculosis patients in health care facilities