Yonsei Med J.
2015 Jan;56(1):112-123. 10.3349/ymj.2015.56.1.112.
The Effect of Bortezomib on Expression of Inflammatory Cytokines and Survival in a Murine Sepsis Model Induced by Cecal Ligation and Puncture
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. jmkim@yuhs.ac
- 2Department of Infectious Diseases, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Microbiology, Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- 4Brain Korea 21 Project for Medical Science, Brain Research Institute and Department of Pharmacology, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2352796
- DOI: http://doi.org/10.3349/ymj.2015.56.1.112
Abstract
- PURPOSE
Although the proteasome inhibitor known as bortezomib can modulate the inflammatory process through the nuclear factor-kappa B signaling pathway, the immunomodulatory effect of pre-incubated bortezomib has not been fully evaluated for inflammation by infectious agents. Therefore, we evaluated the effect of bortezomib on the expression of inflammatory cytokines and mediators in macrophage cell lines and on survival in a murine peritonitis sepsis model.
MATERIALS AND METHODS
Bortezomib was applied 1 hr before lipopolysaccharide (LPS) stimulation in RAW 264.7 cells. The cecal ligation and puncture (CLP) experiments were performed in C57BL/6J mice.
RESULTS
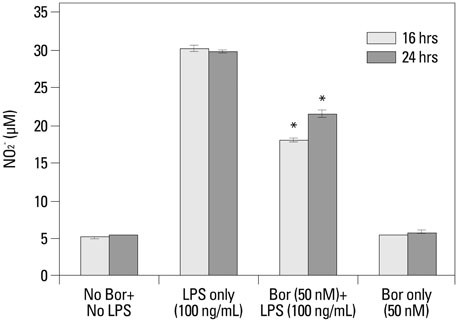
Pre-incubation with bortezomib (25 nM or 50 nM) prior to LPS (50 ng/mL or 100 ng/mL) stimulation significantly recovered the number of viable RAW 264.7 cells compared to those samples without pre-incubation. Bortezomib decreased various inflammatory cytokines as well as nitric oxide production in LPS-stimulated cells. The 7-day survival rate in mice that had received bortezomib at 0.01 mg/kg concentration 1 hr prior to CLP was significantly higher than in the mice that had only received a normal saline solution of 1 mL 1 hr prior to CLP. In addition, the administration of bortezomib at 0.01 mg/kg concentration 1 hr before CLP resulted in a significant decrease in inflammation of the lung parenchyma. Collectively, pretreatment with bortezomib showed an increase in the survival rate and changes in the levels of inflammatory mediators.
CONCLUSION
These results support the possibility of pretreatment with bortezomib as a new therapeutic target for the treatment of overwhelming inflammation, which is a characteristic of severe sepsis.
Keyword
MeSH Terms
-
Animals
Boronic Acids/administration & dosage/pharmacology/*therapeutic use
Cecum/*pathology
Cell Adhesion Molecules/metabolism
Cell Line
Cell Proliferation/drug effects
Cell Survival/drug effects
Chymotrypsin/metabolism
Cytokines/*metabolism
Disease Models, Animal
Inflammation Mediators/*metabolism
Ligation
Lipopolysaccharides/pharmacology
Lung/drug effects/metabolism/pathology
Male
Mice, Inbred C57BL
Nitric Oxide/metabolism
Proteasome Inhibitors/pharmacology
Punctures
Pyrazines/administration & dosage/pharmacology/*therapeutic use
Sepsis/*drug therapy
Boronic Acids
Cell Adhesion Molecules
Chymotrypsin
Cytokines
Inflammation Mediators
Lipopolysaccharides
Proteasome Inhibitors
Pyrazines
Nitric Oxide
Figure
Reference
-
1. Mao X, Pan X, Cheng T, Zhang X. Therapeutic potential of the proteasome inhibitor Bortezomib on titanium particle-induced inflammation in a murine model. Inflammation. 2012; 35:905–912.
Article2. Koca SS, Ozgen M, Dagli F, Tuzcu M, Ozercan IH, Sahin K, et al. Proteasome inhibition prevents development of experimental dermal fibrosis. Inflammation. 2012; 35:810–817.
Article3. Yannaki E, Papadopoulou A, Athanasiou E, Kaloyannidis P, Paraskeva A, Bougiouklis D, et al. The proteasome inhibitor bortezomib drastically affects inflammation and bone disease in adjuvant-induced arthritis in rats. Arthritis Rheum. 2010; 62:3277–3288.
Article4. Blanco B, Sánchez-Abarca LI, Caballero-Velázquez T, Santamaría C, Inogés S, Pérez-Simón JA. Depletion of alloreactive T-cells in vitro using the proteasome inhibitor bortezomib preserves the immune response against pathogens. Leuk Res. 2011; 35:1412–1415.
Article5. Brun J. Proteasome inhibition as a novel therapy in treating rheumatoid arthritis. Med Hypotheses. 2008; 71:65–72.
Article6. Inoue S, Nakase H, Matsuura M, Mikami S, Ueno S, Uza N, et al. The effect of proteasome inhibitor MG132 on experimental inflammatory bowel disease. Clin Exp Immunol. 2009; 156:172–182.
Article7. Lee SW, Kim JH, Park YB, Lee SK. Bortezomib attenuates murine collagen-induced arthritis. Ann Rheum Dis. 2009; 68:1761–1767.
Article8. Palombella VJ, Rando OJ, Goldberg AL, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell. 1994; 78:773–785.
Article9. Paramore A, Frantz S. Bortezomib. Nat Rev Drug Discov. 2003; 2:611–612.
Article10. Meiners S, Ludwig A, Stangl V, Stangl K. Proteasome inhibitors: poisons and remedies. Med Res Rev. 2008; 28:309–327.
Article11. Moehler T, Goldschmidt H. Therapy of relapsed and refractory multiple myeloma. Recent Results Cancer Res. 2011; 183:239–271.
Article12. Tsujimoto H, Ono S, Efron PA, Scumpia PO, Moldawer LL, Mochizuki H. Role of Toll-like receptors in the development of sepsis. Shock. 2008; 29:315–321.
Article13. Christaki E, Anyfanti P, Opal SM. Immunomodulatory therapy for sepsis: an update. Expert Rev Anti Infect Ther. 2011; 9:1013–1033.
Article14. Qureshi N, Perera PY, Shen J, Zhang G, Lenschat A, Splitter G, et al. The proteasome as a lipopolysaccharide-binding protein in macrophages: differential effects of proteasome inhibition on lipopolysaccharide-induced signaling events. J Immunol. 2003; 171:1515–1525.
Article15. Safránek R, Ishibashi N, Oka Y, Ozasa H, Shirouzu K, Holecek M. Modulation of inflammatory response in sepsis by proteasome inhibition. Int J Exp Pathol. 2006; 87:369–372.
Article16. Reis J, Tan X, Yang R, Rockwell CE, Papasian CJ, Vogel SN, et al. A combination of proteasome inhibitors and antibiotics prevents lethality in a septic shock model. Innate Immun. 2008; 14:319–329.
Article17. Jeong SJ, Lim BJ, Park S, Choi D, Kim HW, Ku NS, et al. The effect of sRAGE-Fc fusion protein attenuates inflammation and decreases mortality in a murine cecal ligation and puncture model. Inflamm Res. 2012; 61:1211–1218.
Article18. O'Brien MA, Moravec RA, Riss TL, Bulleit RF. Homogeneous, bioluminescent proteasome assays. Methods Mol Biol. 2008; 414:163–181.19. Ishiyama M, Miyazono Y, Sasamoto K, Ohkura Y, Ueno K. A highly water-soluble disulfonated tetrazolium salt as a chromogenic indicator for NADH as well as cell viability. Talanta. 1997; 44:1299–1305.
Article20. Isobe I, Michikawa M, Yanagisawa K. Enhancement of MTT, a tetrazolium salt, exocytosis by amyloid beta-protein and chloroquine in cultured rat astrocytes. Neurosci Lett. 1999; 266:129–132.
Article21. Lee JW, Choi CH, Choi JJ, Park YA, Kim SJ, Hwang SY, et al. Altered MicroRNA expression in cervical carcinomas. Clin Cancer Res. 2008; 14:2535–2542.
Article22. Kotewicz ML, D'Alessio JM, Driftmier KM, Blodgett KP, Gerard GF. Cloning and overexpression of Moloney murine leukemia virus reverse transcriptase in Escherichia coli. Gene. 1985; 35:249–258.
Article23. Tsikas D. Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: appraisal of the Griess reaction in the L-arginine/nitric oxide area of research. J Chromatogr B Analyt Technol Biomed Life Sci. 2007; 851:51–70.
Article24. Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009; 4:31–36.
Article25. Lutterloh EC, Opal SM, Pittman DD, Keith JC Jr, Tan XY, Clancy BM, et al. Inhibition of the RAGE products increases survival in experimental models of severe sepsis and systemic infection. Crit Care. 2007; 11:R122.
Article26. Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003; 348:138–150.
Article27. Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4th ed. New York: Garland Science;2002.28. Thijs A, Thijs LG. Pathogenesis of renal failure in sepsis. Kidney Int Suppl. 1998; 66:S34–S37.29. Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003; 426:895–899.
Article30. Naujokat C, Hoffmann S. Role and function of the 26S proteasome in proliferation and apoptosis. Lab Invest. 2002; 82:965–980.
Article31. Jesenberger V, Jentsch S. Deadly encounter: ubiquitin meets apoptosis. Nat Rev Mol Cell Biol. 2002; 3:112–121.
Article32. Wang T. The 26S proteasome system in the signaling pathways of TGF-beta superfamily. Front Biosci. 2003; 8:d1109–d1127.
Article33. Wojcikiewicz RJ. Regulated ubiquitination of proteins in GPCR-initiated signaling pathways. Trends Pharmacol Sci. 2004; 25:35–41.
Article34. Muratani M, Tansey WP. How the ubiquitin-proteasome system controls transcription. Nat Rev Mol Cell Biol. 2003; 4:192–201.
Article35. Fuchs SY. The role of ubiquitin-proteasome pathway in oncogenic signaling. Cancer Biol Ther. 2002; 1:337–341.36. Kloetzel PM, Ossendorp F. Proteasome and peptidase function in MHC-class-I-mediated antigen presentation. Curr Opin Immunol. 2004; 16:76–81.
Article37. Bowerman B, Kurz T. Degrade to create: developmental requirements for ubiquitin-mediated proteolysis during early C. elegans embryogenesis. Development. 2006; 133:773–784.
Article38. Elliott PJ, Zollner TM, Boehncke WH. Proteasome inhibition: a new anti-inflammatory strategy. J Mol Med (Berl). 2003; 81:235–245.
Article39. Mattingly LH, Gault RA, Murphy WJ. Use of systemic proteasome inhibition as an immune-modulating agent in disease. Endocr Metab Immune Disord Drug Targets. 2007; 7:29–34.
Article40. Amiri KI, Horton LW, LaFleur BJ, Sosman JA, Richmond A. Augmenting chemosensitivity of malignant melanoma tumors via proteasome inhibition: implication for bortezomib (VELCADE, PS-341) as a therapeutic agent for malignant melanoma. Cancer Res. 2004; 64:4912–4918.
Article41. Fujioka S, Schmidt C, Sclabas GM, Li Z, Pelicano H, Peng B, et al. Stabilization of p53 is a novel mechanism for proapoptotic function of NF-kappaB. J Biol Chem. 2004; 279:27549–27559.
Article42. Sun K, Wilkins DE, Anver MR, Sayers TJ, Panoskaltsis-Mortari A, Blazar BR, et al. Differential effects of proteasome inhibition by bortezomib on murine acute graft-versus-host disease (GVHD): delayed administration of bortezomib results in increased GVHD-dependent gastrointestinal toxicity. Blood. 2005; 106:3293–3299.
Article43. Blanco B, Pérez-Simón JA, Sánchez-Abarca LI, Carvajal-Vergara X, Mateos J, Vidriales B, et al. Bortezomib induces selective depletion of alloreactive T lymphocytes and decreases the production of Th1 cytokines. Blood. 2006; 107:3575–3583.
Article44. Russell JA. Management of sepsis. N Engl J Med. 2006; 355:1699–1713.
Article45. Shen J, Reis J, Morrison DC, Papasian C, Raghavakaimal S, Kolbert C, et al. Key inflammatory signaling pathways are regulated by the proteasome. Shock. 2006; 25:472–484.
Article46. Meiners S, Ludwig A, Lorenz M, Dreger H, Baumann G, Stangl V, et al. Nontoxic proteasome inhibition activates a protective antioxidant defense response in endothelial cells. Free Radic Biol Med. 2006; 40:2232–2241.
Article47. Hassan SW, Doody KM, Hardy S, Uetani N, Cournoyer D, Tremblay ML. Increased susceptibility to dextran sulfate sodium induced colitis in the T cell protein tyrosine phosphatase heterozygous mouse. PLoS One. 2010; 5:e8868.
Article48. Gray PW, Goeddel DV. Cloning and expression of murine immune interferon cDNA. Proc Natl Acad Sci U S A. 1983; 80:5842–5846.
Article49. Shaftel SS, Kyrkanides S, Olschowka JA, Miller JN, Johnson RE, O'Banion MK. Sustained hippocampal IL-1 beta overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J Clin Invest. 2007; 117:1595–1604.
Article50. Moore KW, Vieira P, Fiorentino DF, Trounstine ML, Khan TA, Mosmann TR. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science. 1990; 248:1230–1234.
Article51. Colle JH, Falanga PB, Singer M, Hevin B, Milon G. Quantitation of messenger RNA by competitive RT-PCR: a simplified read out assay. J Immunol Methods. 1997; 210:175–184.
Article52. Fujitani Y, Kanaoka Y, Aritake K, Uodome N, Okazaki-Hatake K, Urade Y. Pronounced eosinophilic lung inflammation and Th2 cytokine release in human lipocalin-type prostaglandin D synthase transgenic mice. J Immunol. 2002; 168:443–449.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sepsis Mortality in CIITA Deficient Mice is Associated with Excessive Release of High-mobility Group Box 1
- Ventilation accelerates lung injury in septic mice
- Time Course of Inducible NOS Expression of Lung Tissue during Sepsis in a Rat Model
- Impact of Exercise Training on Survival Rate and Neural Cell Death in Sepsis Through the Maintenance of Redox Equilibrium
- Long Non-Coding RNA RMRP Contributes to Sepsis-Induced Acute Kidney Injury