Ann Dermatol.
2015 Jun;27(3):250-256. 10.5021/ad.2015.27.3.250.
Tranexamic Acid Diminishes Laser-Induced Melanogenesis
- Affiliations
-
- 1Department of Dermatology, Inje University, Sanggye Paik Hospital, Seoul, Korea.
- 2Department of Dermatology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea. csesnumd@gmail.com
- 3Aesthetic Research Team, Amore Pacific Corporation Research and Development Center, Yongin, Korea.
- KMID: 2352494
- DOI: http://doi.org/10.5021/ad.2015.27.3.250
Abstract
- BACKGROUND
The treatment of post-inflammatory hyperpigmentation (PIH) remains challenging. Tranexamic acid, a well-known anti-fibrinolytic drug, has recently demonstrated a curative effect towards melasma and ultraviolet-induced PIH in Asian countries. However, the precise mechanism of its inhibitory effect on melanogenesis is not fully understood.
OBJECTIVE
In order to clarify the inhibitory effect of tranexamic acid on PIH, we investigated its effects on mouse melanocytes (i.e., melan-a cells) and human melanocytes.
METHODS
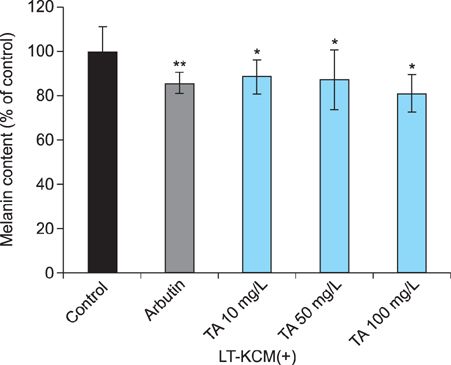
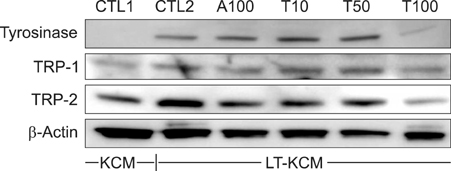
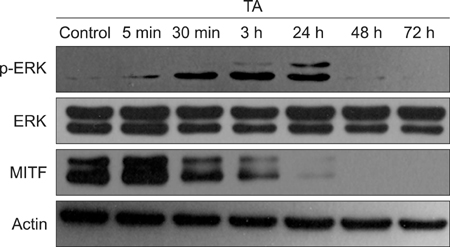
Melan-a cells and human melanocytes were cultured with fractional CO2 laser-treated keratinocyte-conditioned media. Melanin content and tyrosinase activity were evaluated in cells treated with or without tranexamic acid. Protein levels of tyrosinase, tyrosinase-related protein (TRP)-1, and TRP-2 were evaluated in melan-a cells. Signaling pathway molecules involved in melanogenesis in melanoma cells were also investigated.
RESULTS
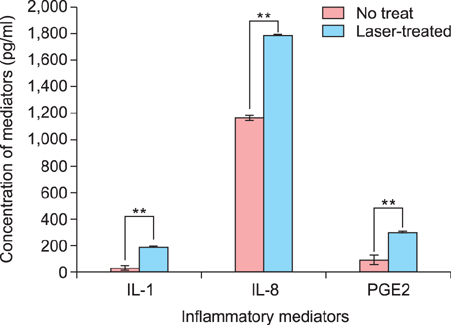
Tranexamic acid-treated melanocytes exhibited reduced melanin content and tyrosinase activity. Tranexamic acid also decreased tyrosinase, TRP-1, and TRP-2 protein levels. This inhibitory effect on melanogenesis was considered to be involved in extracellular signal-regulated kinase signaling pathways and subsequently microphthalmia-associated transcription factor degradation.
CONCLUSION
Tranexamic acid may be an attractive candidate for the treatment of PIH.
Keyword
MeSH Terms
-
Animals
Asian Continental Ancestry Group
Humans
Hyperpigmentation
MART-1 Antigen
Melanins
Melanocytes
Melanoma
Melanosis
Mice
Microphthalmia-Associated Transcription Factor
Monophenol Monooxygenase
Phosphotransferases
Tranexamic Acid*
MART-1 Antigen
Melanins
Microphthalmia-Associated Transcription Factor
Monophenol Monooxygenase
Phosphotransferases
Tranexamic Acid
Figure
Reference
-
1. Gupta AK, Gover MD, Nouri K, Taylor S. The treatment of melasma: a review of clinical trials. J Am Acad Dermatol. 2006; 55:1048–1065.
Article2. Costin GE, Hearing VJ. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J. 2007; 21:976–994.
Article3. Ando H, Kondoh H, Ichihashi M, Hearing VJ. Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J Invest Dermatol. 2007; 127:751–761.
Article4. Lee JH, Park JG, Lim SH, Kim JY, Ahn KY, Kim MY, et al. Localized intradermal microinjection of tranexamic acid for treatment of melasma in Asian patients: a preliminary clinical trial. Dermatol Surg. 2006; 32:626–631.
Article5. Li D, Shi Y, Li M, Liu J, Feng X. Tranexamic acid can treat ultraviolet radiation-induced pigmentation in guinea pigs. Eur J Dermatol. 2010; 20:289–292.
Article6. Maeda K, Naganuma M. Topical trans-4-aminomethylcyclohexanecarboxylic acid prevents ultraviolet radiation-induced pigmentation. J Photochem Photobiol B. 1998; 47:136–141.
Article7. Maeda K, Tomita Y. Mechanism of the inhibitory effect of tranexamic acid on melanogenesis in cultured human melanocytes in the presence of keratinocyte-conditioned medium. J Health Sci. 2007; 53:389–396.
Article8. Horrow JC, Van Riper DF, Strong MD, Brodsky I, Parmet JL. Hemostatic effects of tranexamic acid and desmopressin during cardiac surgery. Circulation. 1991; 84:2063–2070.
Article9. Park HY, Pongpudpunth M, Lee J, Yaar M. Biology of melanocyte. In : Wolff K, Goldsmith LA, I.Katz S, Gilchrest BA, Paller AS, Lefell DJ, editors. Fitzpatrick's dermatology in general medicine. 7th ed. Columbus: McGraw-Hill;2008. p. 591–607.10. Kim DS, Park SH, Kwon SB, Park ES, Huh CH, Youn SW, et al. Sphingosylphosphorylcholine-induced ERK activation inhibits melanin synthesis in human melanocytes. Pigment Cell Res. 2006; 19:146–153.
Article11. Dunn CJ, Goa KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs. 1999; 57:1005–1032.12. Morelli JG, Norris DA. Influence of inflammatory mediators and cytokines on human melanocyte function. J Invest Dermatol. 1993; 100:2 Suppl. 191S–195S.
Article13. Starner RJ, McClelland L, Abdel-Malek Z, Fricke A, Scott G. PGE(2) is a UVR-inducible autocrine factor for human melanocytes that stimulates tyrosinase activation. Exp Dermatol. 2010; 19:682–684.
Article14. Yun WJ, Bang SH, Min KH, Kim SW, Lee MW, Chang SE. Epidermal growth factor and epidermal growth factor signaling attenuate laser-induced melanogenesis. Dermatol Surg. 2013; 39:1903–1911.
Article15. Burd A, Zhu N, Poon VK. A study of Q-switched Nd:YAG laser irradiation and paracrine function in human skin cells. Photodermatol Photoimmunol Photomed. 2005; 21:131–137.
Article16. Chang TS. An updated review of tyrosinase inhibitors. Int J Mol Sci. 2009; 10:2440–2475.
Article17. Hemesath TJ, Price ER, Takemoto C, Badalian T, Fisher DE. MAP kinase links the transcription factor Microphthalmia to c-Kit signalling in melanocytes. Nature. 1998; 391:298–301.
Article18. Wu M, Hemesath TJ, Takemoto CM, Horstmann MA, Wells AG, Price ER, et al. c-Kit triggers dual phosphorylations, which couple activation and degradation of the essential melanocyte factor Mi. Genes Dev. 2000; 14:301–312.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Melasma Showing Response to Combination Therapy with Oral Tranexamic Acid and the Q-Switched Nd:YAG Laser
- Is irrational use of tranexamic acid justified in anesthesia practice?
- Treatment Effect of Tranexamic Acid in Plasma D-dimer Level Elevated Anti-histamine Resistant Chronic Urticaria Patients
- Efficacy of Tranexamic Acid during Primary Total Knee Arthroplasty: Comparative Study between Intravenous Use and Topical Use
- What Are the Optimal Dose of Administration and Time of Drainage for Topical Tranexamic Acid in Patients Undergoing Cardiac Surgery?