J Korean Med Sci.
2015 Oct;30(Suppl 1):S19-S24. 10.3346/jkms.2015.30.S1.S19.
Data Management and Site-Visit Monitoring of the Multi-Center Registry in the Korean Neonatal Network
- Affiliations
-
- 1Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea.
- 2Department of Pediatrics, Seoul National University Bundang Hospital, Seongnam, Korea.
- 3Department of Pediatrics, Ajou University School of Medicine, Suwon, Korea. drparkms@ajou.ac.kr
- KMID: 2351132
- DOI: http://doi.org/10.3346/jkms.2015.30.S1.S19
Abstract
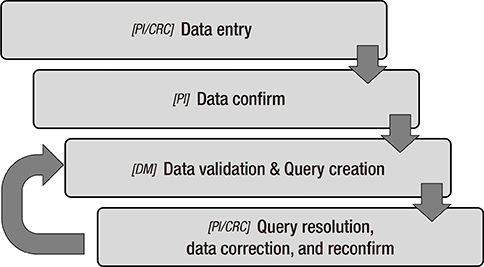
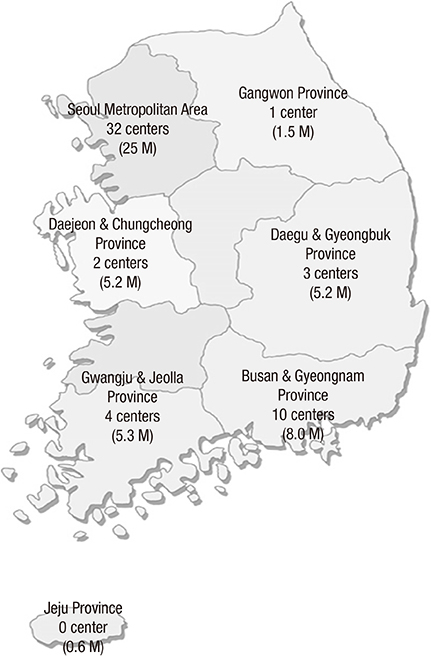
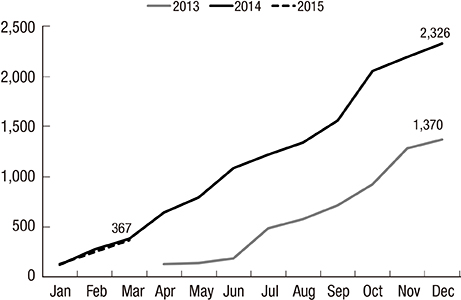
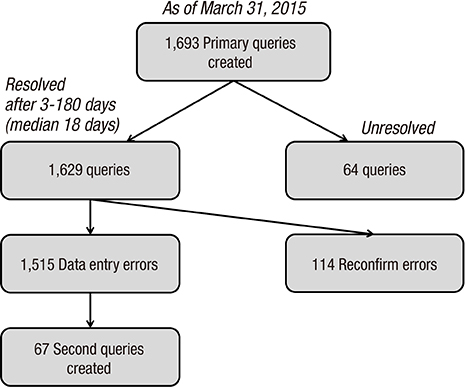
- The Korean Neonatal Network (KNN), a nationwide prospective registry of very-low-birth-weight (VLBW, < 1,500 g at birth) infants, was launched in April 2013. Data management (DM) and site-visit monitoring (SVM) were crucial in ensuring the quality of the data collected from 55 participating hospitals across the country on 116 clinical variables. We describe the processes and results of DM and SVM performed during the establishment stage of the registry. The DM procedure included automated proof checks, electronic data validation, query creation, query resolution, and revalidation of the corrected data. SVM included SVM team organization, identification of unregistered cases, source document verification, and post-visit report production. By March 31, 2015, 4,063 VLBW infants were registered and 1,693 queries were produced. Of these, 1,629 queries were resolved and 64 queries remain unresolved. By November 28, 2014, 52 participating hospitals were visited, with 136 site-visits completed since April 2013. Each participating hospital was visited biannually. DM and SVM were performed to ensure the quality of the data collected for the KNN registry. Our experience with DM and SVM can be applied for similar multi-center registries with large numbers of participating centers.
MeSH Terms
Figure
Cited by 1 articles
-
Future of neonatology in Korea: the way forward
Yun Sil Chang
J Korean Med Assoc. 2016;59(7):506-513. doi: 10.5124/jkma.2016.59.7.506.
Reference
-
1. Chang YS, Ahn SY, Park WS. Committee on Program and Planning and Advisory Committee of Korean Neonatal Network. The establishment of the Korean Neonatal Network (KNN). Neonatal Med. 2013; 20:169–178.2. Thakkar M, O'Shea M. The role of neonatal networks. Semin Fetal Neonatal Med. 2006; 11:105–110.3. Prokscha S. Edit checks. In : Prokscha S, editor. Practical guide to clinical data management. 3rd ed. Boca Raton: CRC Press Taylor & Francis Group;2012. p. 37–42.4. Prokscha S. Cleaning data. In : Prokscha S, editor. Practical guide to clinical data management. 3rd ed. Boca Raton: CRC Press Taylor & Francis Group;2012. p. 73–84.5. Seol HC. Guideline for electronic data processing and management in clinical trial. Cheongju: Ministry of Food and Drug Safety;2012.6. The Executive Committee of Korean Neonatal Network. 2013 Korean Neonatal Network Annual Report. Cheongwon: Korean Centers for Disease Control and Prevention;2014.