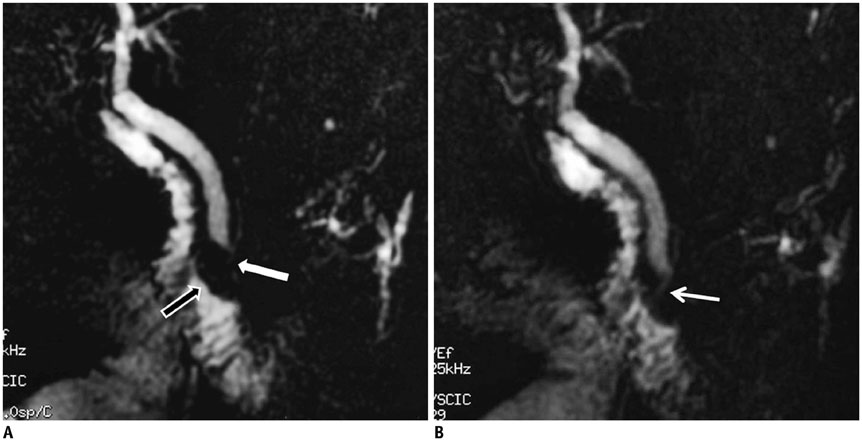
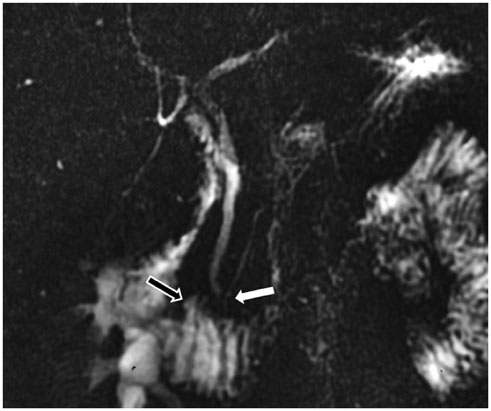
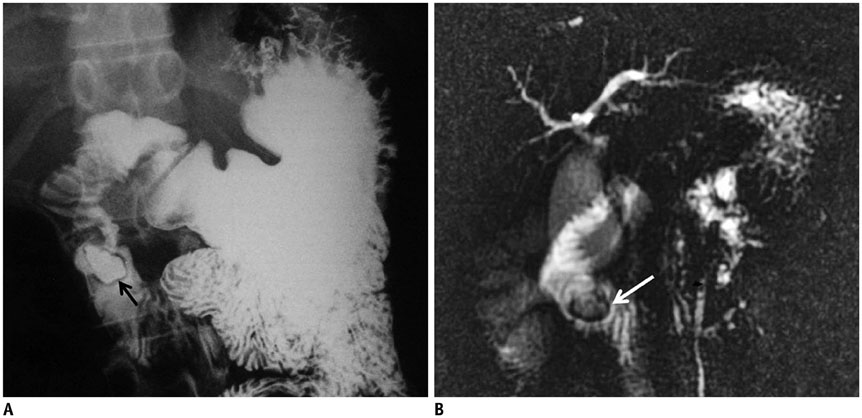
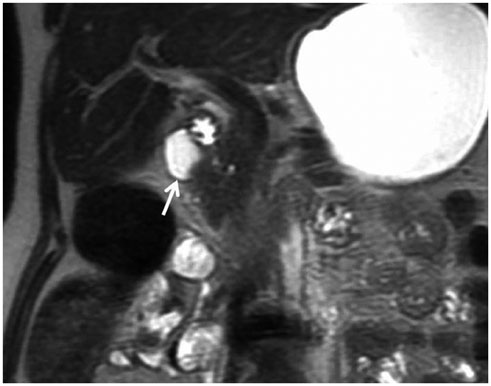
Korean J Radiol.
2015 Dec;16(6):1240-1252. 10.3348/kjr.2015.16.6.1240.
MRI Findings of Intrinsic and Extrinsic Duodenal Abnormalities and Variations
- Affiliations
-
- 1Department of Radiology, Ankara University School of Medicine, Ankara 06100, Turkey. ebrumd2001@yahoo.com
- 2Department of Gastroenterology, Ankara University School of Medicine, Ankara 06100, Turkey.
- KMID: 2344277
- DOI: http://doi.org/10.3348/kjr.2015.16.6.1240
Abstract
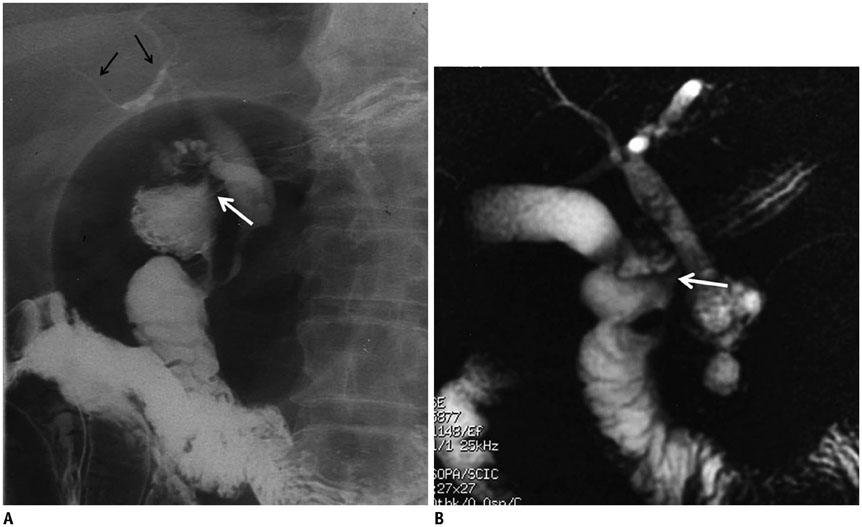
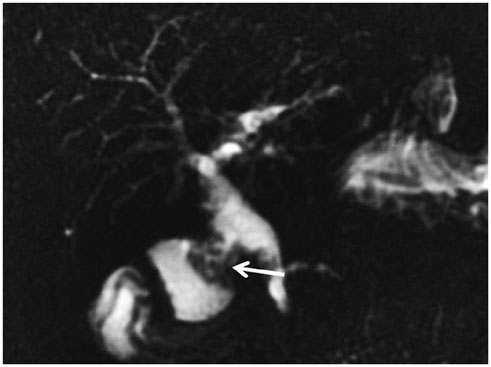
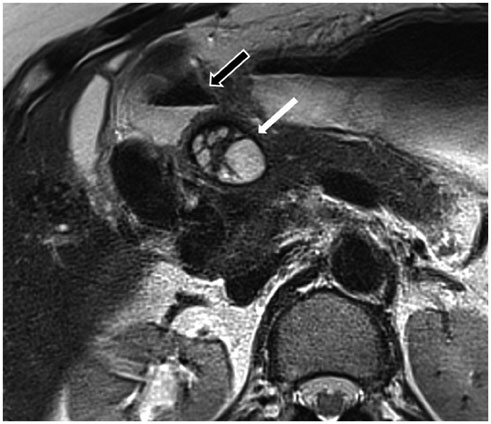
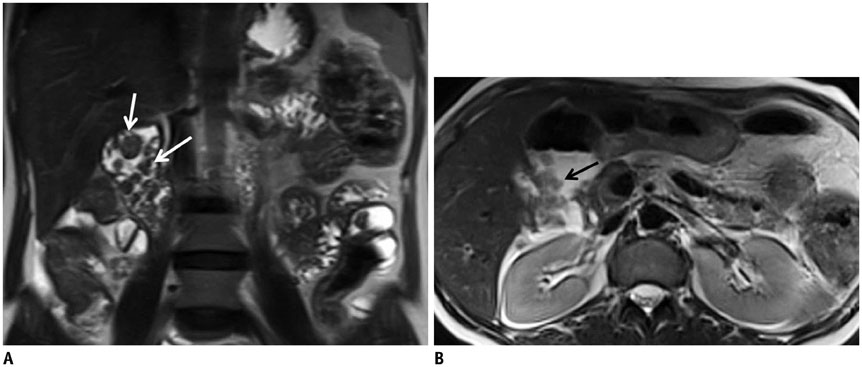
- This pictorial review aims to illustrate the magnetic resonance imaging (MRI) findings and presentation patterns of anatomical variations and various benign and malignant pathologies of the duodenum, including sphincter contraction, major papilla variation, prominent papilla, diverticulum, annular pancreas, duplication cysts, choledochocele, duodenal wall thickening secondary to acute pancreatitis, postbulbar stenosis, celiac disease, fistula, choledochoduodenostomy, external compression, polyps, Peutz-Jeghers syndrome, ampullary carcinoma and adenocarcinoma. MRI is a useful imaging tool for demonstrating duodenal pathology and its anatomic relationships with adjacent organs, which is critical for establishing correct diagnosis and planning appropriate treatment, especially for surgery.
Keyword
MeSH Terms
-
Ampulla of Vater/anatomy & histology/radiography
Choledochal Cyst/pathology/radiography
Diverticulum/radiography
Duodenal Diseases/pathology/*radiography
Duodenum/*anatomy & histology/radiography
Humans
*Magnetic Resonance Imaging
Pancreas/abnormalities/anatomy & histology/radiography
Pancreatic Diseases/radiography
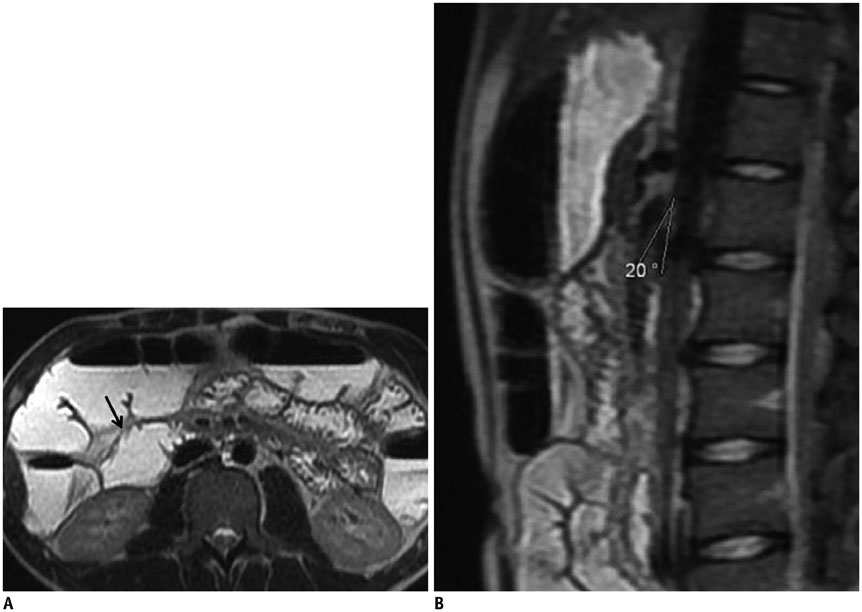
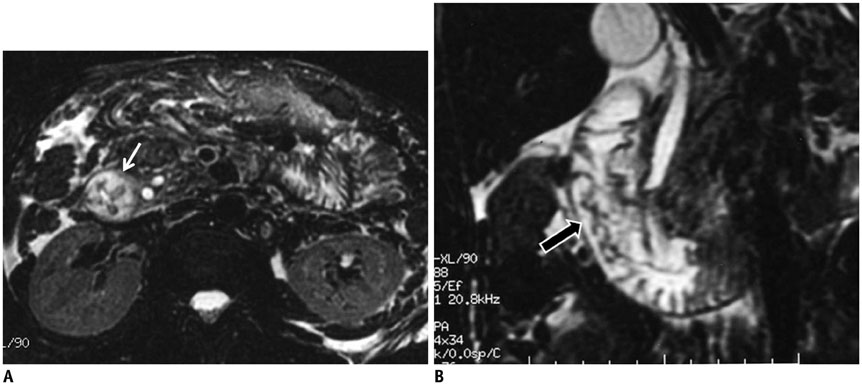
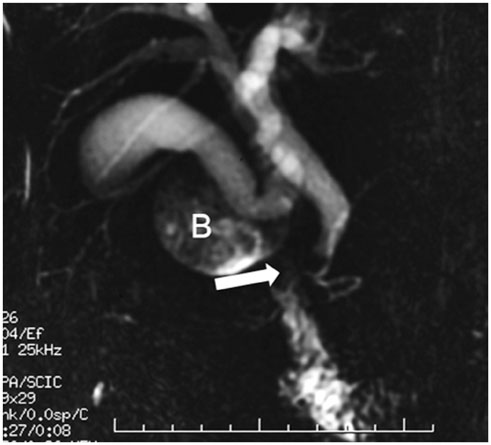
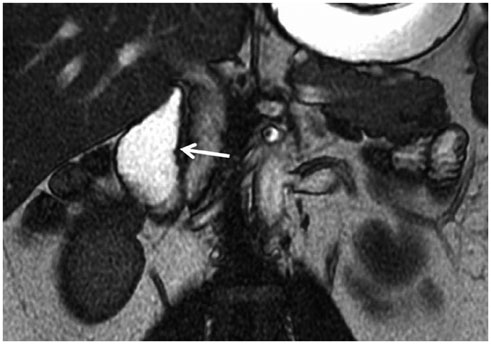
Figure
Reference
-
1. Van Hoe L, Gryspeerdt S, Vanbeckevoort D, De Jaegere T, Van Steenbergen W, Dewandel P, et al. Normal Vaterian sphincter complex: evaluation of morphology and contractility with dynamic single-shot MR cholangiopancreatography. AJR Am J Roentgenol. 1998; 170:1497–1500.2. Staritz M. Pharmacology of the sphincter of Oddi. Endoscopy. 1988; 20:Suppl 1. 171–174.3. Horiguchi S, Kamisawa T. Major duodenal papilla and its normal anatomy. Dig Surg. 2010; 27:90–93.4. Kim S, Lee NK, Lee JW, Kim CW, Lee SH, Kim GH, et al. CT evaluation of the bulging papilla with endoscopic correlation. Radiographics. 2007; 27:1023–1038.5. Kim JH, Kim MJ, Chung JJ, Lee WJ, Yoo HS, Lee JT. Differential diagnosis of periampullary carcinomas at MR imaging. Radiographics. 2002; 22:1335–1352.6. Cronin CG, Lohan DG, DeLappe E, Roche C, Murphy JM. Duodenal abnormalities at MR small-bowel follow-through. AJR Am J Roentgenol. 2008; 191:1082–1092.7. Jayaraman MV, Mayo-Smith WW, Movson JS, Dupuy DE, Wallach MT. CT of the duodenum: an overlooked segment gets its due. Radiographics. 2001; 21:S147–S160.8. Zissin R, Osadchy A, Gayer G, Shapiro-Feinberg M. Pictorial review. CT of duodenal pathology. Br J Radiol. 2002; 75:78–84.9. Mazziotti S, Costa C, Ascenti G, Gaeta M, Pandolfo A, Blandino A. MR cholangiopancreatography diagnosis of juxtapapillary duodenal diverticulum simulating a cystic lesion of the pancreas: usefulness of an oral negative contrast agent. AJR Am J Roentgenol. 2005; 185:432–435.10. Hwang JI, Chiang JH, Yu C, Cheng HC, Chang CY, Mueller PR. Pictorial review: Radiological diagnosis of duodenal abnormalities. Clin Radiol. 1998; 53:323–332.11. Tsitouridis I, Emmanouilidou M, Goutsaridou F, Kokozidis G, Kalambakas A, Papastergiou C, et al. MR cholangiography in the evaluation of patients with duodenal periampullary diverticulum. Eur J Radiol. 2003; 47:154–160.12. Takamatsu S, Gabata T, Matsui O, Noto M, Ninomiya I, Nonomura A. Intraluminal duodenal diverticulum: MR findings. Abdom Imaging. 2006; 31:39–42.13. Tu AS, Tran MH, Larsen CR. CT-appearance of intraluminal duodenal diverticulum. The "halo" sign. Comput Med Imaging Graph. 1998; 22:81–83.14. Afridi SA, Fichtenbaum CJ, Taubin H. Review of duodenal diverticula. Am J Gastroenterol. 1991; 86:935–938.15. Tasu JP, Rocher L, Amouyal P, Lorand I, Rondeau Y, Buffet C, et al. Intraluminal duodenal diverticulum: radiological and endoscopic ultrasonography findings of an unusual cause of acute pancreatitis. Eur Radiol. 1999; 9:1898–1900.16. Finnie IA, Ghosh P, Garvey C, Poston GJ, Rhodes JM. Intraluminal duodenal diverticulum causing recurrent pancreatitis: treatment by endoscopic incision. Gut. 1994; 35:557–559.17. Berrocal T, Torres I, Gutiérrez J, Prieto C, del Hoyo ML, Lamas M. Congenital anomalies of the upper gastrointestinal tract. Radiographics. 1999; 19:855–872.18. Lee NK, Kim S, Jeon TY, Kim HS, Kim DH, Seo HI, et al. Complications of congenital and developmental abnormalities of the gastrointestinal tract in adolescents and adults: evaluation with multimodality imaging. Radiographics. 2010; 30:1489–1507.19. Sandrasegaran K, Patel A, Fogel EL, Zyromski NJ, Pitt HA. Annular pancreas in adults. AJR Am J Roentgenol. 2009; 193:455–460.20. Türkvatan A, Erden A, Türkoğlu MA, Yener Ö. Congenital variants and anomalies of the pancreas and pancreatic duct: imaging by magnetic resonance cholangiopancreaticography and multidetector computed tomography. Korean J Radiol. 2013; 14:905–913.21. Macpherson RI. Gastrointestinal tract duplications: clinical, pathologic, etiologic, and radiologic considerations. Radiographics. 1993; 13:1063–1080.22. Rice CA, Anderson TM, Sepahdari S. Computed tomography and ultrasonography of carcinoma in duplication cysts. J Comput Assist Tomogr. 1986; 10:233–235.23. Can MF, Kaymakc¸ioğlu N, Yağci G, Görgülü S, Tufan T. An adult choledochocele case presented with gastric outlet obstruction: a rare presentation. Turk J Gastroenterol. 2006; 17:70–73.24. Jabłońska B. Biliary cysts: etiology, diagnosis and management. World J Gastroenterol. 2012; 18:4801–4810.25. Lee HK, Park SJ, Yi BH, Lee AL, Moon JH, Chang YW. Imaging features of adult choledochal cysts: a pictorial review. Korean J Radiol. 2009; 10:71–80.26. Konen E, Amitai M, Apter S, Garniek A, Gayer G, Nass S, et al. CT angiography of superior mesenteric artery syndrome. AJR Am J Roentgenol. 1998; 171:1279–1281.27. Gustafsson L, Falk A, Lukes PJ, Gamklou R. Diagnosis and treatment of superior mesenteric artery syndrome. Br J Surg. 1984; 71:499–501.28. Kennedy KV, Yela R, Achalandabaso Mdel M, Martín-Pérez E. Superior mesenteric artery syndrome: diagnostic and therapeutic considerations. Rev Esp Enferm Dig. 2013; 105:236–238.29. Felton BM, White JM, Racine MA. An uncommon case of abdominal pain: superior mesenteric artery syndrome. West J Emerg Med. 2012; 13:501–502.30. Bauer S, Karplus R, Belsky V, Mha HA. Superior mesenteric artery syndrome: a forgotten entity. Isr Med Assoc J. 2013; 15:189–191.31. Al-Rashedy M, El-Dhuwaib Y, Issa M, Ballester P, Ammori BJ. Laparoscopic management of acquired benign duodenal strictures in adults. Internet J Surg. 2005; 6:14.32. Lee HJ, Ha HK, Kim MH, Jeong YK, Kim PN, Lee MG, et al. ERCP and CT findings of ectopic drainage of the common bile duct into the duodenal bulb. AJR Am J Roentgenol. 1997; 169:517–520.33. Dadzan E, Akhondi H. Choledochoduodenal fistula presenting with pneumobilia in a patient with gallbladder cancer: a case report. J Med Case Rep. 2012; 6:61.34. Okabe T, Ohwada S, Ogawa T, Takeyoshi I, Sato Y, Kamoshita N, et al. Gallbladder carcinoma with choledochoduodenal fistula: a case report with surgical treatment. Hepatogastroenterology. 1999; 46:1660–1663.35. Aramaki M, Ikeda M, Kawanaka H, Nishijima N, Tsutsumi N, Kano T, et al. Choledochoduodenostomy: simple side-to-side anastomosis. J Hepatobiliary Pancreat Surg. 2000; 7:486–488.36. McCowin MJ, Federle MP. Computed tomography of pancreatic pseudocysts of the duodenum. AJR Am J Roentgenol. 1985; 145:1003–1007.37. Bellon EM, George CR, Schreiber H, Marshall JB. Pancreatic pseudocysts of the duodenum. AJR Am J Roentgenol. 1979; 133:827–831.38. Yano T, Yamamoto H. Vascular, polypoid, and other lesions of the small bowel. Best Pract Res Clin Gastroenterol. 2009; 23:61–74.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Annular Pancreas with a Duodenal Web: a Rare Presentation with Simultaneous Intrinsic and Extrinsic Duodenal Obstruction
- A Preliminary Study for the Development and the Standardization of Korean Version of the Intrinsic/Extrinsic Religious Orientation Scale
- Production of interleukin 4 and interferon gamma in CD8+ T cells from patients with intrinsic and extrinsic asthma
- Age-related Changes in the Frequency of Intrinsic and Extrinsic Atopic Dermatitis: A Single-center Retrospective Study
- Vascular Anatomy of Spinal Cord