J Korean Foot Ankle Soc.
2014 Sep;18(3):100-107. 10.14193/jkfas.2014.18.3.100.
Comparison of Efficiency of Self-renewal and Differentiation Potential in Tendon-derived Mesenchymal Stem Cells Isolated by Magnetic-activated Cell Sorting Method or Colony Picking Method
- Affiliations
-
- 1Department of Orthopaedic Surgery, Yonsei University College of Medicine, Seoul, Korea. OSMEDIC@yuhs.ac
- 2Brain Korea 21 PLUS Project for Medical Science, Yonsei University, Seoul, Korea.
- KMID: 2333472
- DOI: http://doi.org/10.14193/jkfas.2014.18.3.100
Abstract
- PURPOSE
The purpose of this study is to evaluate the efficacy of mesenchymal stem cell (MSC) isolation by the magnetic-activated cell sorting (MACS) method in tendon tissue-derived cells compared to the colony picking method for isolation of MSCs by picking colonyforming cells.
MATERIALS AND METHODS
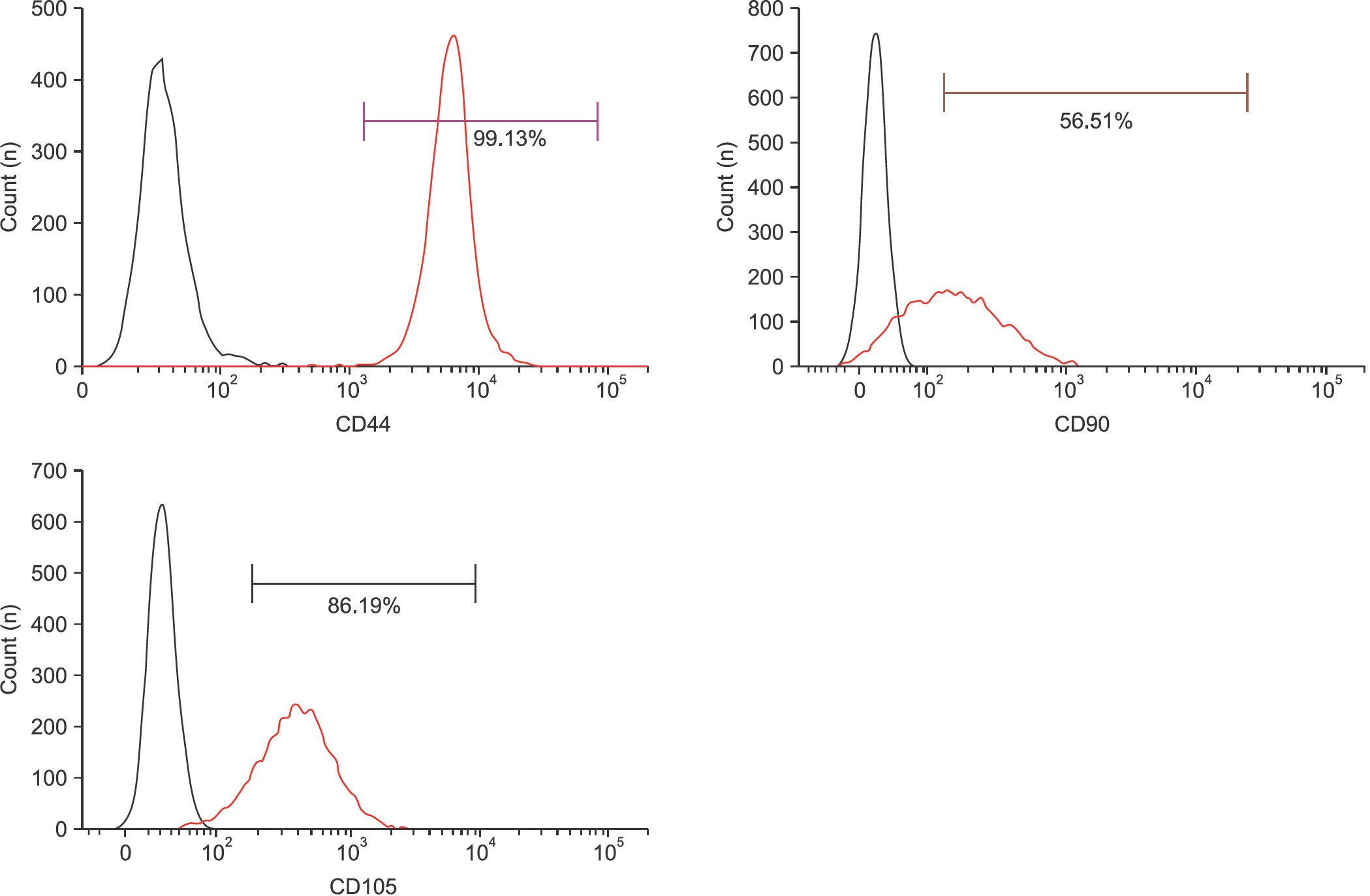
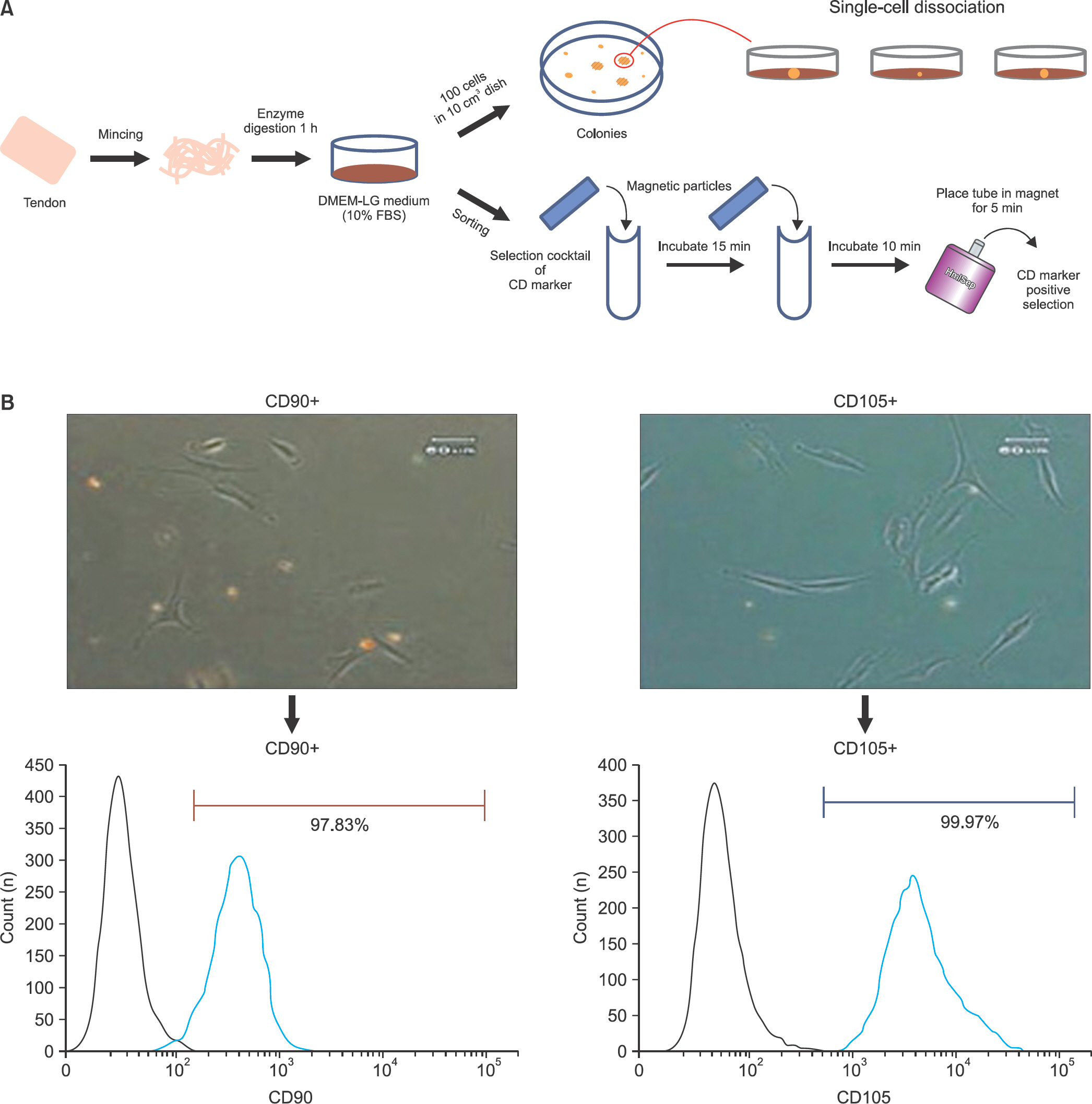
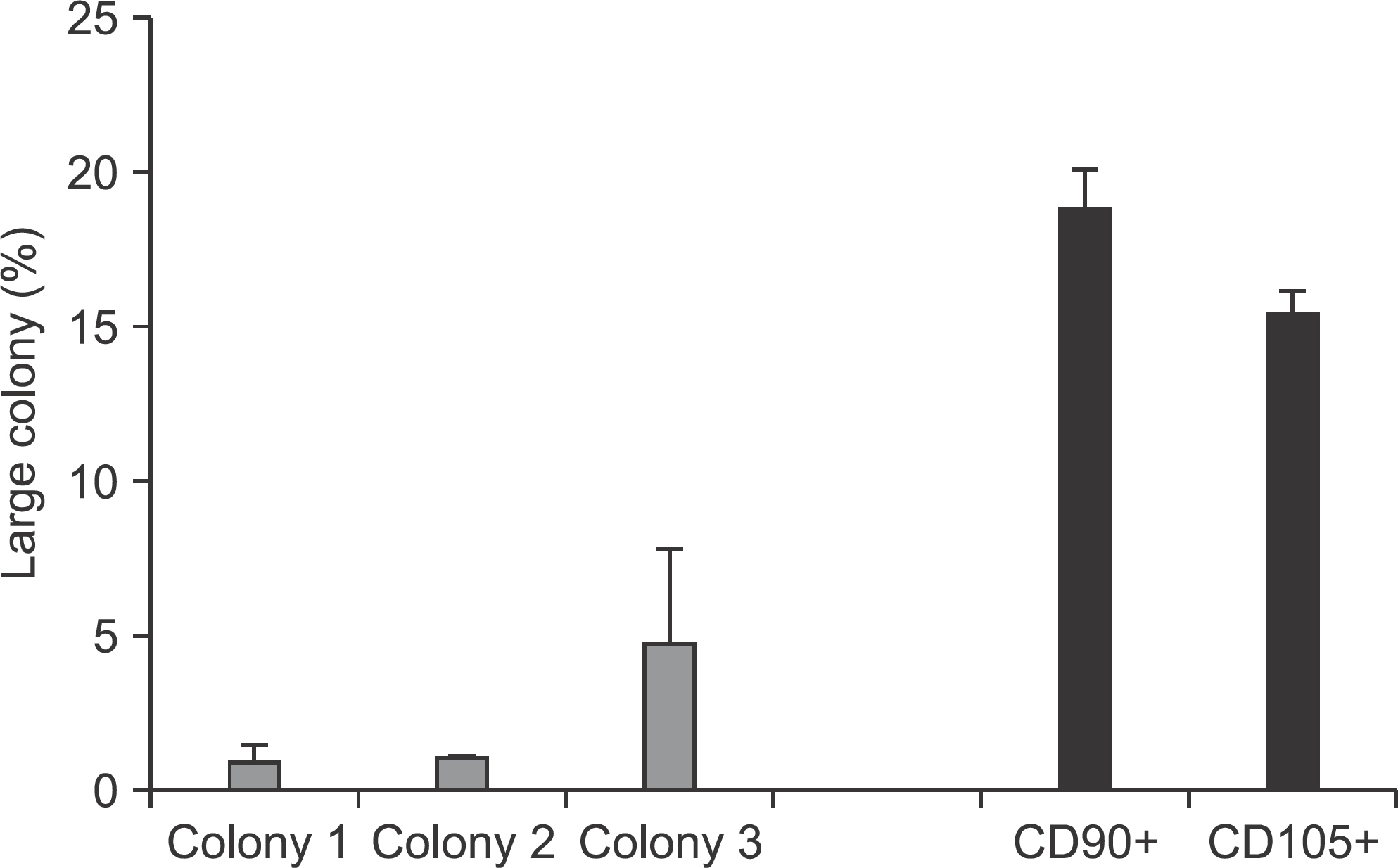
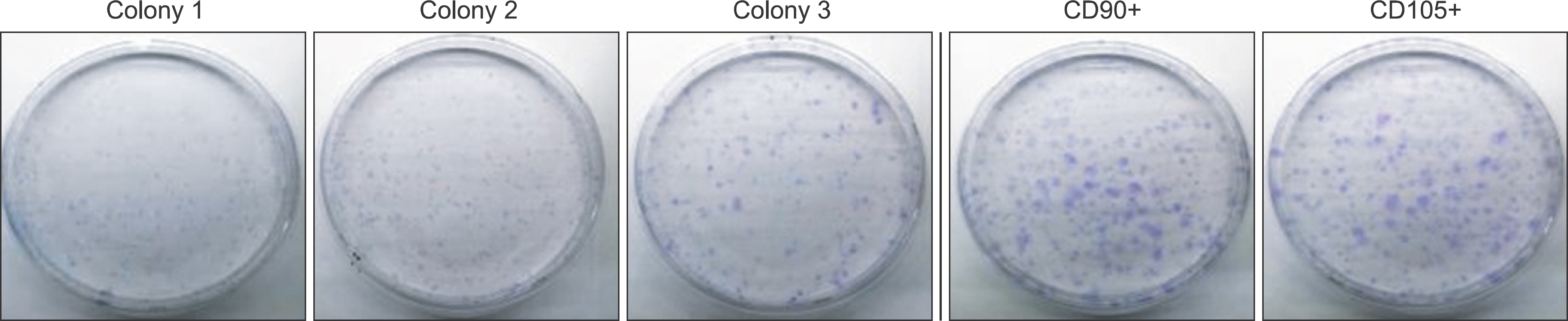
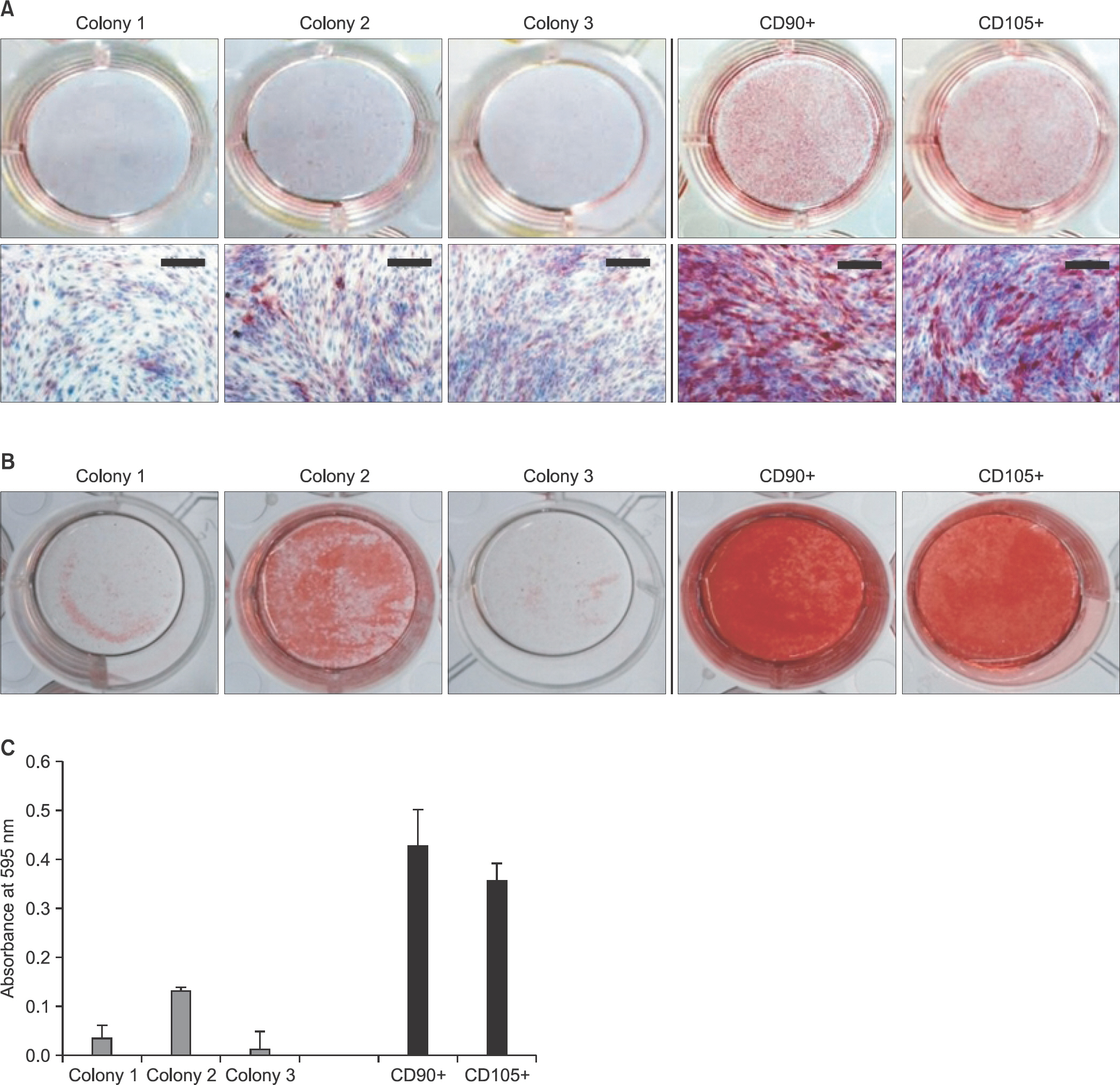
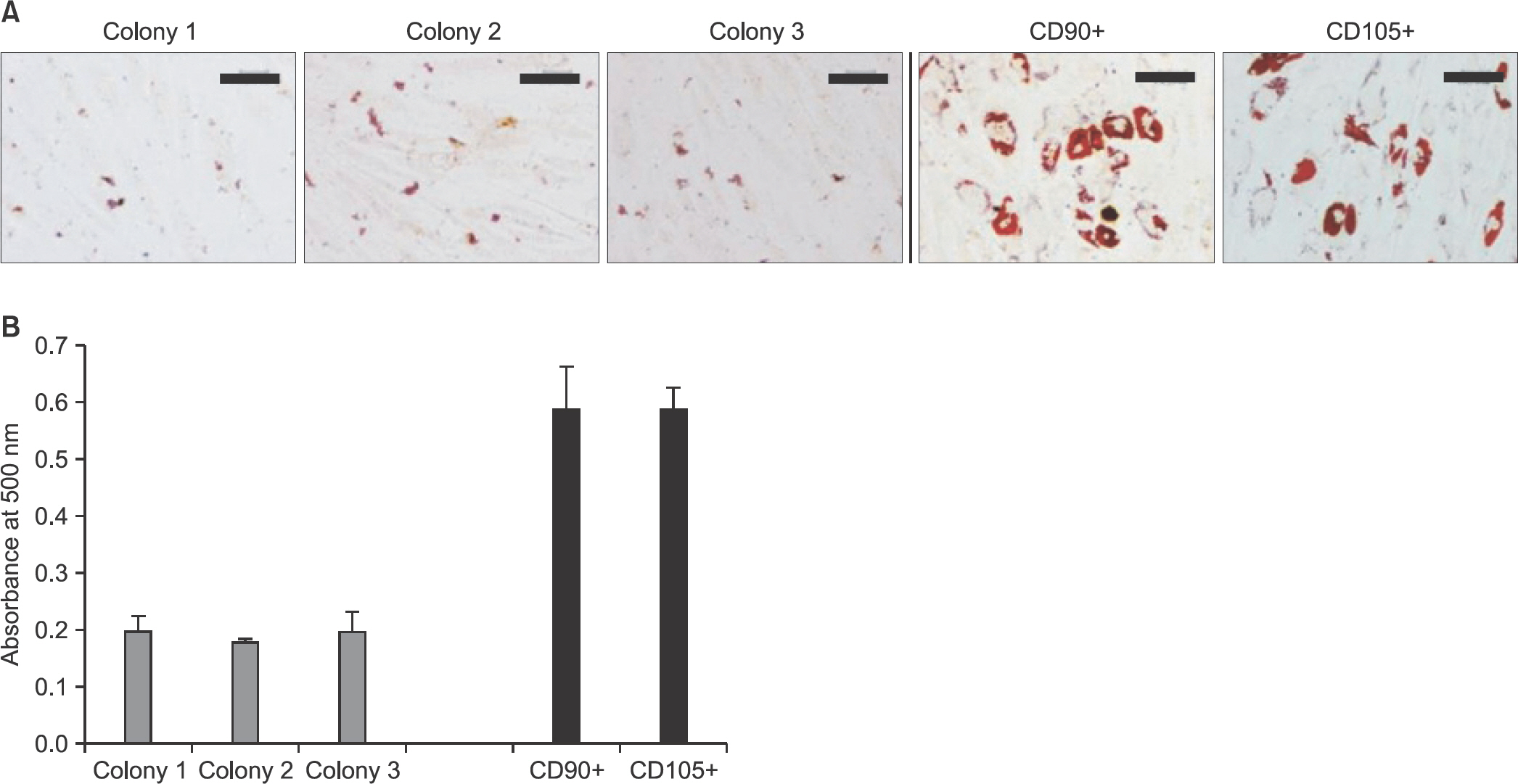
Human tendon-derived cells were isolated by enzyme digestion using normal tendon tissues from three donors. We used the magnetic kit and well-known MSC markers (CD90 or CD105) to isolate MSCs in tendon-derived cells using MACS. Cloning cylinders were used to isolate colony-forming cells having MSC characteristics in tendon-derived cells. Colony-forming unitfibroblast (CFU-F) assay was used to evaluate the self-renewal capacity of cells isolated using the colony picking method or MACS. For comparison of differentiation potentials into osteogenic or adipogenic lineage between two groups, alizarin red S and oil red O staining were performed at 14 days after induction of differentiation in vitro.
RESULTS
Flow cytometry results showed that early passage tendon-derived cells expressed CD44 in 99.13%, CD90 in 56.51%, and CD105 in 86.19%. In the CFU-F assay, CD90+ or CD105+ cells isolated with MACS showed larger colony formation in size than cells isolated using the colony picking method. We also observed that CD90+ or CD105+ cells were constantly differentiated into both osteogenic and adipogenic lineages in cells from all donors, whereas cells isolated using the colony picking method were heterogeneous in differentiation potentials to the osteogenic and adipogenic lineages.
CONCLUSION
CD90+ or CD105+ cells isolated using MACS showed superior MSC characteristics in the self-renewal and multi-differentiation capacities compared with cells isolated using the colony picking method.
MeSH Terms
Figure
Reference
-
1.Butler DL., Juncosa N., Dressler MR. Functional efficacy of tendon repair processes. Annu Rev Biomed Eng. 2004. 6:303–29.
Article2.Rees JD., Wilson AM., Wolman RL. Current concepts in the management of tendon disorders. Rheumatology (Oxford). 2006. 41:108–21.
Article3.Riley G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology (Oxford). 2004. 43:131–42.
Article4.Yao L., Bestwick CS., Bestwick LA., Maffulli N., Aspden RM. Phenotypic drift in human tenocyte culture. Tissue Eng. 2006. 12:1843–9.
Article5.Awad HA., Boivin GP., Dressler MR., Smith FN., Young RG., Butler DL. Repair of patellar tendon injuries using a cell-collagen composite. J Orthop Res. 2003. 21:420–31.
Article6.Chen X., Song XH., Yin Z., Zou XH., Wang LL., Hu H, et al. Stepwise differentiation of human embryonic stem cells promotes tendon regeneration by secreting fetal tendon matrix and differentiation factors. Stem Cells. 2009. 27:1276–87.
Article7.Harris MT., Butler DL., Boivin GP., Florer JB., Schantz EJ., Wenstrup RJ. Mesenchymal stem cells used for rabbit tendon repair can form ectopic bone and express alkaline phosphatase activity in constructs. J Orthop Res. 2004. 22:998–1003.
Article8.Bi Y., Ehirchiou D., Kilts TM., Inkson CA., Embree MC., Sonoyama W, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007. 13:1219–27.
Article9.Tan Q., Lui PP., Rui YF., Wong YM. Comparison of potentials of stem cells isolated from tendon and bone marrow for musculoskeletal tissue engineering. Tissue Eng Part A. 2012. 18:840–11.
Article10.Zhang J., Li B., Wang JH. The role of engineered tendon matrix in the stemness of tendon stem cells in vitro and the promotion of tendon-like tissue formation in vivo. Biomaterials. 2011. 32:6972–81.11.Lui PP., Wong OT. Tendon stem cells: experimental and clinical perspectives in tendon and tendon-bone junction repair. Muscles Ligaments Tendons J. 2012. 2:163–8.12.Zhang J., Wang JH. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskelet Disord. 2010. 11:10.
Article13.Zhang J., Wang JH. Human tendon stem cells better maintain their stemness in hypoxic culture conditions. PLoS One. 2013. 8:e61424.
Article14.Bühring HJ., Battula VL., Treml S., Schewe B., Kanz L., Vogel W. Novel markers for the prospective isolation of human MSC. Ann N Y Acad Sci. 2007. 1106:262–71.15.Gothard D., Dawson JI., Oreffo RO. Assessing the potential of colony morphology for dissecting the CFU-F population from human bone marrow stromal cells. Cell Tissue Res. 2013. 312:237–47.
Article16.Anzalone R., Lo Iacono M., Corrao S., Magno F., Loria T., Cappello F, et al. New emerging potentials for human Wharton’s jelly mesenchymal stem cells: immunological features and hepatocyte-like differentiative capacity. Stem Cells Dev. 2010. 19:423–38.
Article17.Liu F., Akiyama Y., Tai S., Maruyama K., Kawaguchi Y., Muramatsu K, et al. Changes in the expression of CD106, osteogenic genes, and transcription factors involved in the osteogenic differentiation of human bone marrow mesenchymal stem cells. J Bone Miner Metab. 2008. 26:312–20.
Article18.Zhu H., Mitsuhashi N., Klein A., Barsky LW., Weinberg K., Barr ML, et al. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells. 2006. 24:928–31.
Article19.Quintanilla RH Jr., Asprer JS., Vaz C., Tanavde V., Lakshmipathy U. CD44 is a negative cell surface marker for pluripotent stem cell identification during human fibroblast reprogramming. PLoS One. 2014. 9:e81419.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stem cell properties of cells derived from canine periodontal ligament
- Do the Fibroblasts Contained in Early Passage MSC Population Adversely Affect the Characteristics of Stem Cell Population Obtained from Human Placenta?
- A Simple Method to Isolate and Expand Human Umbilical Cord Derived Mesenchymal Stem Cells: Using Explant Method and Umbilical Cord Blood Serum
- Comparative characteristic study from bone marrow-derived mesenchymal stem cells
- Epidural Fat-Derived Mesenchymal Stem Cell: First Report of Epidural Fat-Derived Mesenchymal Stem Cell