J Korean Diabetes Assoc.
2007 Sep;31(5):402-409.
Antibodies to GAD and ICA in Type 2 DM with Secondary Failure of Oral Hypoglycemic Therapy
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, Yeungnam University.
Abstract
-
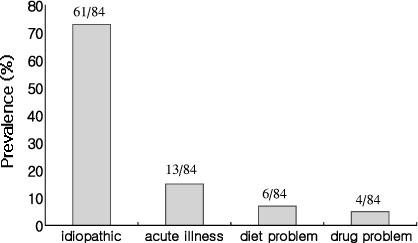
BACKGROUND: Secondary failure of oral hypoglycemic agents is defined as that blood glucose is no longer controlled with sulfonylurea after a proven period of good glycemic control. There are many causes of secondary failure, including that drug problem, acute illnesses, inappropriate drug dosages, oxidative stress & glucose toxicity of beta-cell, etc. And many studies have suggested role of immunologic process such as islet cell antibody (ICA) and/or glutamic acid decarboxylase antibody (GADA) for the causes of secondary failure. So we evaluated the prevalence of ICA & GADA in type 2 diabetes with secondary failure of oral hypoglycemic agents and the pathogenesis of the secondary failure.
METHODS
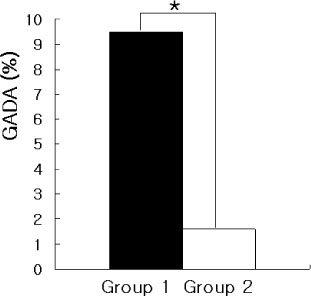
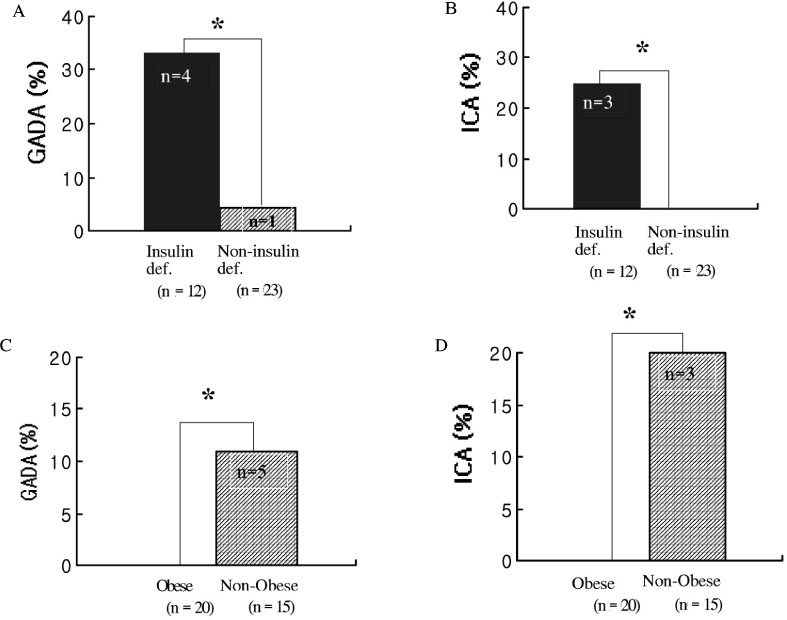
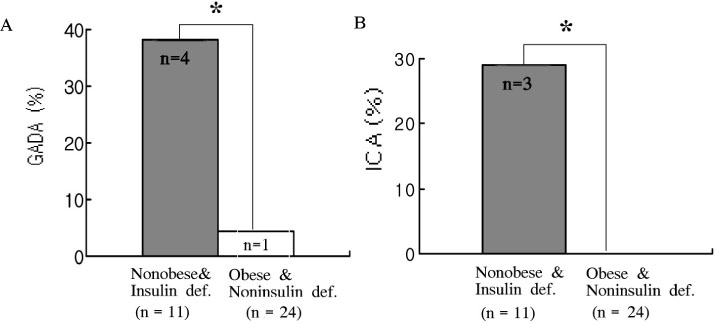
We studied 267 patients with type 2 diabetes. We regarded 84 patients who could not control HbA1c less than 8% after good glycemic control for at least 1 year as secondary failure group (group 1) and regarded the other 183 patients as group 2. We measured GADA in both group, and measured the prevalence of GADA and ICA in secondary failure group who were especially divided into obese group and nonobese group according to BMI and were divided into insulin deficiency group and noninsulin deficiency group according to fasting C-peptide level.
RESULTS
The prevalence of GADA in all subjects was 4.1%, which was 9.5% in group 1 and 1.6% in group 2 (P < 0.05). Among 35 patients of the group 1 who could be checked ICA, the prevalence of GADA & ICA were 33% & 25% in insulin deficiency group and 4.3% & 0% in non-insulin deficiency group, respectively (P < 0.05). The prevalence of GADA & ICA were none in obese group and 33.3% & 20% in nonobese group, respectively (P < 0.05). The prevalence of GADA & ICA were 36.4% & 27.3% in nonobese and insulin deficiency, 4.2% & 0% in obese and non-insulin deficiency.
CONCLUSION
We suggest that autoimmune mechanism is associated with increased risk for secondary failure of oral hypoglycemic agents in type 2 diabetes, so the measurement of GADA and ICA could help to predict the potential risk and insulin treatment.
Keyword
MeSH Terms
Figure
Reference
-
1. DeLawter DW, Moss JM, Tyroler S, Canary JJ. Secondary failure of response to tolbutamide treatment. JAMA. 1959. 171:1786–1792.2. U.K. Prospective Diabetes Study Group. U.K. prospective diabetes study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. Diabetes. 1995. 44:1249–1258.3. Matthews DR, Cull CA, Stratton IM, Holman RR, Turner RC. UK Prospective Diabetes Study (UKPDS) Group. UKPDS 26: Sulphonylurea failure in non-insulin-dependent diabetic patients over six years. Diabet Med. 1998. 15:297–303.4. Fukui M, Nakano M, Shigeta H, Yoshimori K, Fujii M, Kitagawa Y, Mori H, Kajiyama S, Nakamura N, Abe N, Obayashi H, Fukui I, Ohta K, Ohta M, Kondo M. Antibodies to Glutamic Acid Decarboxylase in Japanese Diabetic Patients with Secondary Failure of Oral Hypoglycaemic Therapy. Diabet Med. 1997. 14:148–152.5. Retiene K, Petzoldt R, Althoff-Zucher C, Beyer J, Schoffling K. Clinical studies on glibenclamide. Horm Metab Res. 1969. 1:Suppl. 5–60.6. Shuman CR. Glipizide: an overview. Am J Med. 1983. 75:55–59.7. Salo S, Groop L. Combination of two sulfonylureas. Does it make sense? Diabetes Care. 1988. 11:751–753.8. Stenman S, Melander A, Groop PH, Groop LC. What is the benefit of increasing the sulfonylurea dose? Ann Intern Med. 1993. 118:169–172.9. Rustenbeck I. Desensitization of insulin secretion. Biochem Pharmacol. 2002. 63:1921–1935.10. Krauss S, Zhang CY, Scorrano L, Dalgaard LT, St-Pierre J, Grey ST, Lowell BB. Superoxide-mediated activation of uncoupling protein 2 causes pancreatic beta cell dysfunction. J Clin Invest. 2003. 112:1831–1842.11. Yki-Jarvinen H. Glucose toxicity. Endocr Rev. 1992. 13:415–431.12. Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002. 110:851–860.13. Sesti G, Marini MA, Cardellini M, Sciacqua A, Frontoni S, Andreozzi F, Irace C, Lauro D, Gnasso A, Federici M, Perticone F, Lauro R. The Arg972 variant in insulin receptor substrate-1 is associated with an increased risk of secondary failure to sulfonylurea in patients with type 2 diabetes. Diabetes Care. 2004. 27:1394–1398.14. Groop L, Miettinen A, Groop PH, Meri S, Koskimies S, Bottazzo GF. Organ-specific autoimmunity and HLA-DR antigens as markers for β-cell destruction in patients with type 2 diabetes. Diabetes. 1988. 37:99–103.15. Pittman WB, Acton RT, Barger BO, Bell DS, Go RCP, Murphy CC. HLA-A, -B, and -DR associations in type 1 diabetes mellitus with onset after age forty. Diabetes. 1982. 31:122–125.16. Zavala AV, Fabiano de Bruno LE, Cardoso AI, Mota AH, Capucchio M, Poskus E, Faiunboim L, Basabe JC. Cellular and humoral autoimmunity markers in type 2(non-insulin-dependent) diabetic patients with secondary drug failure. Diabetologia. 1992. 35:1159–1164.17. Irvine WJ, McCallum CJ, Gray RS, Duncan LJP. Clinical and pathogenic significance of pancreatic-islet-cell antibodies in diabetics treated with oral hypoglycemic agents. Lancet. 1977. 1:1025–1027.18. Tuomi T, Groop LC, Zimmet PZ, Rowley MJ, Knowles W, Mackay IR. Antibodies to glutamic acid decarboxylase reveal latent autoimmune diabetes mellitus in adults with a non-insulin-dependent onset of disease. Diabetes. 1993. 42:359–362.19. Krall LP, Bradley RF. "Secondary failure" in the treatment of diabetes mellitus with tolbutamide and with phenformin. Diabetes. 1962. 11:Suppl. 88–93.20. Pontiroli AE, Calderara A, Maffi P, Bonisolli L, Carenini A, Piatti PM, Monti LD, Gallus G, Pozza G, Illeni MT. Secondary failure to oral hypoglycaemic agents in non-obese patients with non-insulin-dependent diabetes is related to reduced insulin release. Diabetes Metab. 1989. 15:79–84.21. Pontiroli AE, Calderara A, Pozza G. Secondary failure of oral hypoglycemic agents: frequency, possible causes, and management. Diabetes Metab Rev. 1994. 10:31–43.22. Lyons TJ, Kennedy L, Atkinson AB, Buchanan KD, Hadden DR, Weaver JA. Predicting the need for insulin therapy in late onset (40-69 years) diabetes mellitus. Diabet Med. 1984. 1:105–107.23. Di Mario U, Irvine WJ, Borsey DQ, Kyner JL, Weston J, Galfo C. Immune abnormalities in diabetic patients not requiring insulin at diagnosis. Diabetologia. 1983. 25:392–395.24. Niskanen LK, Karjalainen J, Sarlund H, Siitonen O, Uusitupa M. Five-year follow-up of islet cell antibodies in type 2(non-insulin dependent) diabetes mellitus. Diabetologia. 1991. 34:402–408.25. Groop LC, Groop PH, Koskimies S. Relationship between β-cell function and HLA antigens in patients with type 2(non-insulin-dependent) diabetes. Diabetologia. 1986. 29:757–760.26. Zimmet PZ, Tuomi T, Mackay IR, Rowley MJ, Knowles W, Cohen M, Lang DA. Latent autoimmune diabetes mellitus in adults(LADA): the role of antibodies to glutamic acid decarboxylase in diagnosis and prediction of insulin dependency. Diabet Med. 1994. 11:299–303.27. Irvine WJ, Sawers JSA, Feck CM, Prescott AJ, Duncan LPJ. The value of islet cell antibody in predicting secondary failure of oral hypoglycemic agent therapy in diabetes mellitus. J Clin Lab Immunol. 1979. 2:23–26.28. Gleishmann H, Zorcher B, Greulich B, Gries FA, Henrichs HR, Bortrams J, Kolb H. Correlation of islet cell antibodies and HLA-DR phenotype with diabetes mellitus in adults. Diabetologia. 1984. 27:90–92.29. Turner R, Stratton I, Horton V, Manley S, Zimmet P, Mackay IR, Shattock M, Bottazzo GF, Holman R. UKPDS 25 : autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. Lancet. 1997. 350:1288–1293.30. Willis JA, Scott RS, Brown LJ, Fobes LV, Schidli RS, Zimmet PZ, Mackay IR, Rowley MJ. Islet cell antibodies and antibodies against glutamic acid decarboxylase in newly diagnosed adult-onset diabetes mellitus. Diabetes Res Clin Pract. 1996. 33:89–97.31. Gottsäter A, Landin-Olsson M, Lernmark A, Fernlund P, Sundkvist G, Hagopian WA. Glutamate decarboxylase antibody levels predict rate of β-cell decline in adult-onset diabetes. Diabetes Res Clin Pract. 1995. 27:133–140.33. Bonifacio E, Genoverse S, Braghi S. Islet autoantibody markers in IDDM: risk assessment strategies yielding high sensitivity. Diabetologia. 1995. 38:816–822.34. Riley WJ, Maclaren NK, Krischer J. A prospective study of the development of diabetes in relatives of patients with insulin-dependent diabetes. N Engl J Med. 1990. 323:1167–1172.35. Insulin-dependent? Lancet. 1985. 2:809–810.36. Gray RS, Irvine WJ, Cameron EHD, Duncan LJP. Glucose and insulin responses to oral glucose in over non-insulin-dependent diabetic subjects with and without the islet cell antibody. Diabetes. 1980. 29:312–316.37. Tuomilehto J, Zimmer P, Mackay R, Koskela P, Vidgren G, Toivanen L, Tuomilehto-Wolf E, Kohtamaki K, Stengard J, Rowley MJ. Antibodies to glutamic acid decarboxylase as predictors of insulin-dependent diabetes mellitus before clinical onset of disease. Lancet. 1994. 343:1383–1385.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of the Persistence of Islet Cell Cytoplasmic Antibodies and Glutamic Acid Decarboxylase ( GAD ) 65 Antibodies in Type 1 Diabetic Children
- Mesurement of GAD Antibodies using Radioligand Binding Assay, IRMA and RIA in Patients with Tye 1 Diabetes Mellitus
- Chronic Complications in Adult Diabetic Patients with and without GAD Antibody
- Use of Oral Hypoglycemic Agents in Type 2 Diabetic Patients with Hepatic Dysfunction
- Prevalence of Autoimmune Antibodies in Type I Diabetic Children and Their Siblings