J Periodontal Implant Sci.
2015 Apr;45(2):62-68. 10.5051/jpis.2015.45.2.62.
Histomorphometric analysis of microcrack healing after the installation of mini-implants
- Affiliations
-
- 1Department of Orthodontics and Dental Research Institute, Seoul National University School of Dentistry, Seoul, Korea. drortho@snu.ac.kr
- KMID: 2329657
- DOI: http://doi.org/10.5051/jpis.2015.45.2.62
Abstract
- PURPOSE
The goal of this study was to investigate the histomorphometric characteristics of the healing process of microcracks in the cortical bone after the installation of mini-implants (MIs).
METHODS
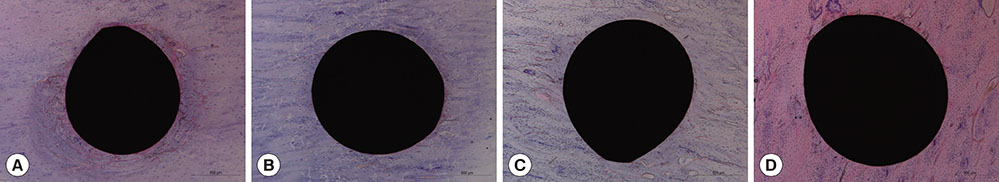
Self-drilling MIs were inserted into the tibial diaphysis of twelve adult male New Zealand rabbits. Four MIs per rabbit were placed randomly. The animals were divided into four groups according to the length of the healing period: group A was sacrificed immediately, group B was sacrificed after one week, group C was sacrificed after two weeks, and group D was sacrificed after four weeks. Cortical bone thickness was measured using micro-computed tomography, and histomorphometric analyses of the cumulative length of the microcracks (CLCr) and the total number of microcracks (NCr) were performed using hematoxylin and eosin staining.
RESULTS
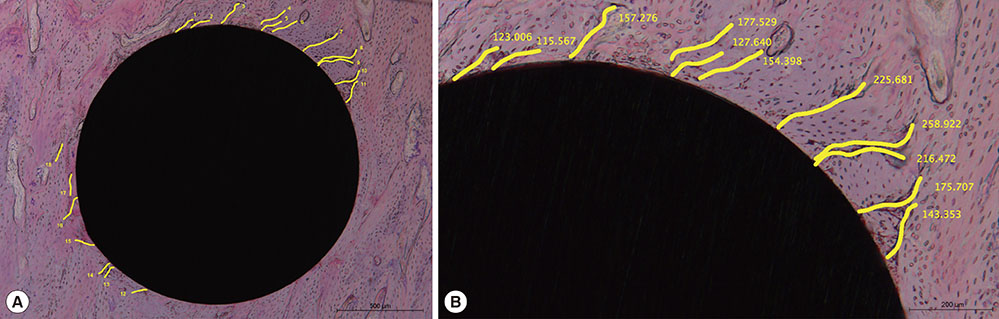
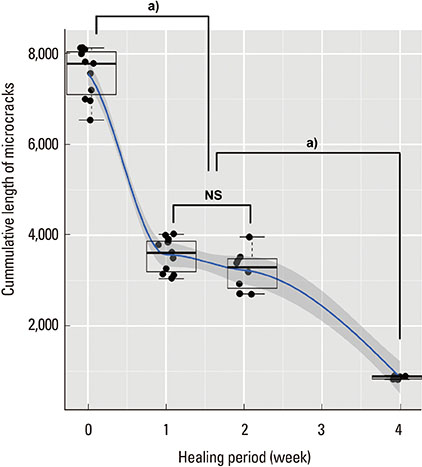
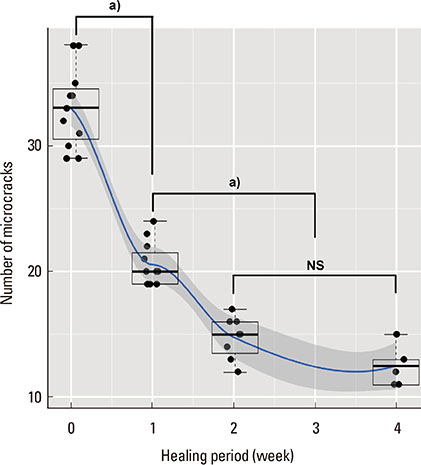
The microcracks were radially and concentrically aligned in the peri-MI bone. The CLCr decreased significantly one week after the surgery, mainly due to healing of the concentrically aligned microcracks. The CLCr showed another significant decrease from two weeks after the surgery to four weeks after the surgery, mainly reflecting healing of the radially aligned microcracks. A statistically significant decrease in the NCr occurred as the microcracks healed from zero weeks to two weeks. However, no significant difference in the NCr was found between groups C and D.
CONCLUSIONS
In order to improve the primary stability of MIs, delayed loading and a healing period of a certain length are recommended to ensure the optimal healing of microcracks and bone remodeling.
MeSH Terms
Figure
Reference
-
1. Proffit WR, Fields HW. Mechanical principles in orthodontic force control. Contemporary orthodontics. 3rd ed. St. Louis (MO): Mosby;2000. p. 326–362.2. Carano A, Velo S, Leone P, Siciliani G. Clinical applications of the Miniscrew Anchorage System. J Clin Orthod. 2005; 39:9–24.3. Miyawaki S, Koyama I, Inoue M, Mishima K, Sugahara T, Takano-Yamamoto T. Factors associated with the stability of titanium screws placed in the posterior region for orthodontic anchorage. Am J Orthod Dentofacial Orthop. 2003; 124:373–378.
Article4. Cheng SJ, Tseng IY, Lee JJ, Kok SH. A prospective study of the risk factors associated with failure of mini-implants used for orthodontic anchorage. Int J Oral Maxillofac Implants. 2004; 19:100–106.5. Park HS, Jeong SH, Kwon OW. Factors affecting the clinical success of screw implants used as orthodontic anchorage. Am J Orthod Dentofacial Orthop. 2006; 130:18–25.
Article6. Wiechmann D, Meyer U, Büchter A. Success rate of mini- and micro-implants used for orthodontic anchorage: a prospective clinical study. Clin Oral Implants Res. 2007; 18:263–267.
Article7. Brinley CL, Behrents R, Kim KB, Condoor S, Kyung HM, Buschang PH. Pitch and longitudinal fluting effects on the primary stability of miniscrew implants. Angle Orthod. 2009; 79:1156–1161.
Article8. Florvaag B, Kneuertz P, Lazar F, Koebke J, Zöller JE, Braumann B, et al. Biomechanical properties of orthodontic miniscrews. An in-vitro study. J Orofac Orthop. 2010; 71:53–67.
Article9. Cha JY, Kil JK, Yoon TM, Hwang CJ. Miniscrew stability evaluated with computerized tomography scanning. Am J Orthod Dentofacial Orthop. 2010; 137:73–79.
Article10. Lee NK, Baek SH. Effects of the diameter and shape of orthodontic mini-implants on microdamage to the cortical bone. Am J Orthod Dentofacial Orthop. 2010; 138:8.e1–8.e8.
Article11. Moon SH, Um HS, Lee JK, Chang BS, Lee MK. The effect of implant shape and bone preparation on primary stability. J Periodontal Implant Sci. 2010; 40:239–243.
Article12. Bartold PM, Kuliwaba JS, Lee V, Shah S, Marino V, Fazzalari NL. Influence of surface roughness and shape on microdamage of the osseous surface adjacent to titanium dental implants. Clin Oral Implants Res. 2011; 22:613–618.
Article13. Shin SY, Shin SI, Kye SB, Chang SW, Hong J, Paeng JY, et al. Bone cement grafting increases implant primary stability in circumferential cortical bone defects. J Periodontal Implant Sci. 2015; 45:30–35.
Article14. Liu SS, Cruz-Marroquin E, Sun J, Stewart KT, Allen MR. Orthodontic mini-implant diameter does not affect in-situ linear microcrack generation in the mandible or the maxilla. Am J Orthod Dentofacial Orthop. 2012; 142:768–773.
Article15. Herman BC, Cardoso L, Majeska RJ, Jepsen KJ, Schaffler MB. Activation of bone remodeling after fatigue: differential response to linear microcracks and diffuse damage. Bone. 2010; 47:766–772.
Article16. Chapurlat RD, Delmas PD. Bone microdamage: a clinical perspective. Osteoporos Int. 2009; 20:1299–1308.
Article17. Carter DR, Hayes WC. Compact bone fatigue damage: a microscopic examination. Clin Orthop Relat Res. 1977; 265–274.18. Lee TC, Mohsin S, Taylor D, Parkesh R, Gunnlaugsson T, O'Brien FJ, et al. Detecting microdamage in bone. J Anat. 2003; 203:161–172.
Article19. Taing-Watson E, Katona TR, Stewart KT, Ghoneima A, Chu GT, Kyung HM, et al. Microdamage generation by tapered and cylindrical mini-screw implants after pilot drilling. Angle Orthod. Forthcoming 2014.
Article20. Yadav S, Upadhyay M, Liu S, Roberts E, Neace WP, Nanda R. Microdamage of the cortical bone during mini-implant insertion with self-drilling and self-tapping techniques: a randomized controlled trial. Am J Orthod Dentofacial Orthop. 2012; 141:538–546.
Article21. Zar JH. Biostatistical analysis. 2nd ed. Englewood Cliffs (NJ): Prentice Hall International;1984.22. Chugh T, Ganeshkar SV, Revankar AV, Jain AK. Quantitative assessment of interradicular bone density in the maxilla and mandible: implications in clinical orthodontics. Prog Orthod. 2013; 14:38.
Article23. Wang X, Mabrey JD, Agrawal CM. An interspecies comparison of bone fracture properties. Biomed Mater Eng. 1998; 8:1–9.24. Martin RB. Osteonal remodeling in response to screw implantation in canine femora. J Orthop Res. 1987; 5:445–452.25. Huja SS, Katona TR, Burr DB, Garetto LP, Roberts WE. Microdamage adjacent to endosseous implants. Bone. 1999; 25:217–222.
Article26. Eraslan O, Inan O. The effect of thread design on stress distribution in a solid screw implant: a 3D finite element analysis. Clin Oral Investig. 2010; 14:411–416.
Article27. Wu J, Bai YX, Wang BK. Biomechanical and histomorphometric characterizations of osseointegration during mini-screw healing in rabbit tibiae. Angle Orthod. 2009; 79:558–563.
Article28. Zhang L, Zhao Z, Li Y, Wu J, Zheng L, Tang T. Osseointegration of orthodontic micro-screws after immediate and early loading. Angle Orthod. 2010; 80:354–360.
Article29. Wang L, Ye T, Deng L, Shao J, Qi J, Zhou Q, et al. Repair of microdamage in osteonal cortical bone adjacent to bone screw. PLoS One. 2014; 9:e89343.
Article30. Schaffler MB. Role of bone turnover in microdamage. Osteoporos Int. 2003; 14:Suppl 5. S73–S77.
Article31. Hazenberg JG, Freeley M, Foran E, Lee TC, Taylor D. Microdamage: a cell transducing mechanism based on ruptured osteocyte processes. J Biomech. 2006; 39:2096–2103.
Article32. Martin RB. Targeted bone remodeling involves BMU steering as well as activation. Bone. 2007; 40:1574–1580.
Article33. Parfitt AM. The mechanism of coupling: a role for the vasculature. Bone. 2000; 26:319–323.
Article34. Eriksen EF. Cellular mechanisms of bone remodeling. Rev Endocr Metab Disord. 2010; 11:219–227.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- COMPARISON OF RESONANCE FREQUENCY ANALYSIS BETWEEN VARIOUS SURFACE PROPERTIES
- Osseointegration of Magnesium-Incorporated Sand-Blasted Acid-Etched Implant in the Dog Mandible: Resonance Frequency Measurements and Histomorphometric Analysis
- The Influence of the Initial Stability After Dental Implant Installation on the Osseointegration
- Effects of different oxidized surfaces of implant on osseointegration; resonance frequency and histomorphometric analysis study in mini-pigs
- The effect of loading time on the stability of mini-implant