Tuberc Respir Dis.
2015 Apr;78(2):137-141. 10.4046/trd.2015.78.2.137.
Low Grade Pulmonary Lymphomatoid Granulomatosis with an Endobronchial Mass
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 2Division of Pulmonary Medicine, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. gpdush@hanmail.net
- KMID: 2320608
- DOI: http://doi.org/10.4046/trd.2015.78.2.137
Abstract
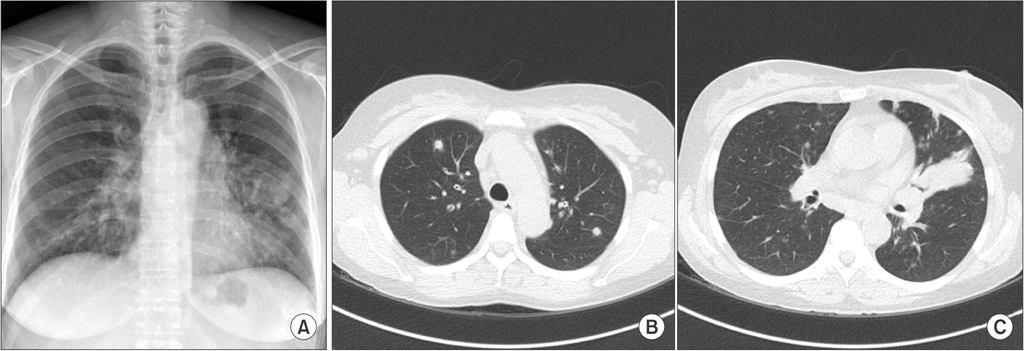
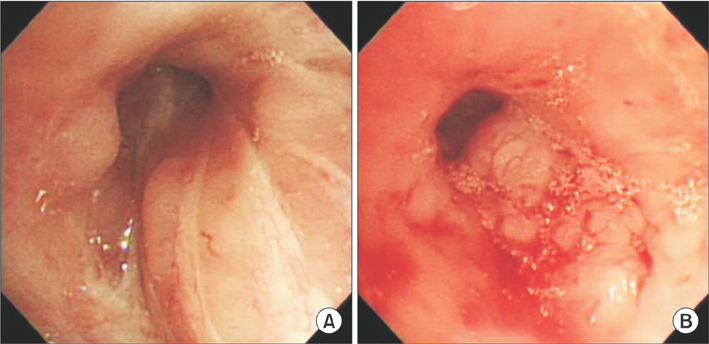
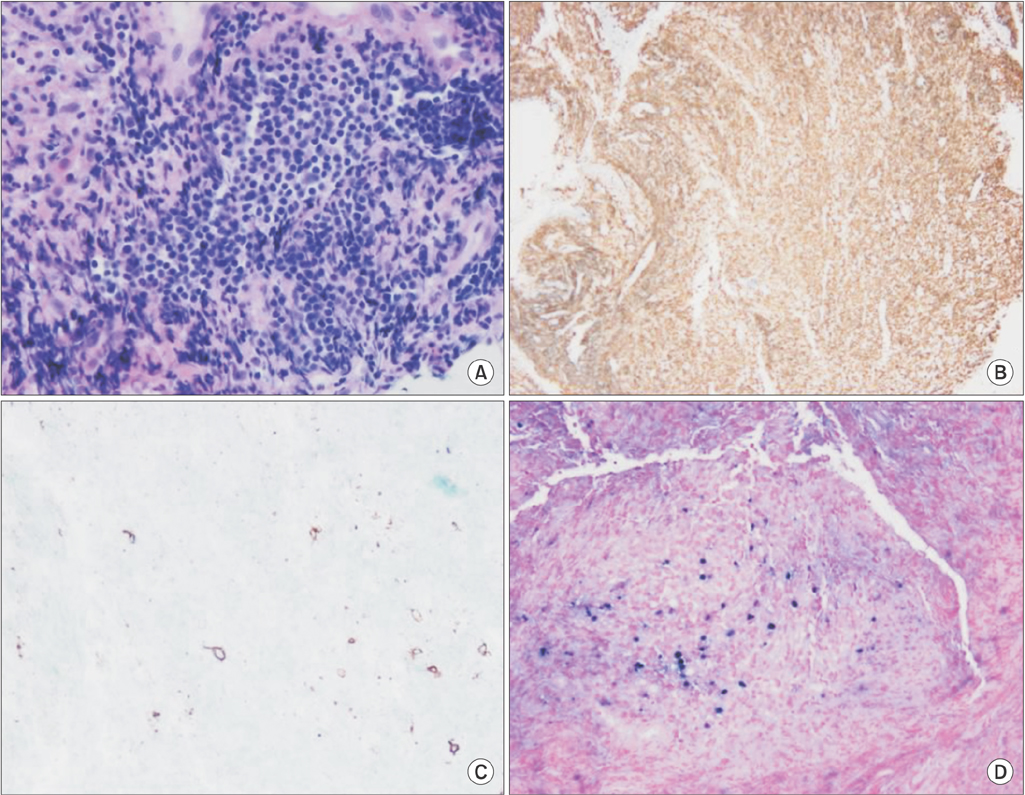
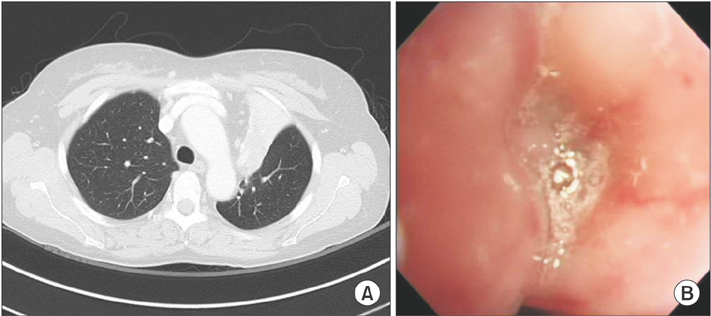
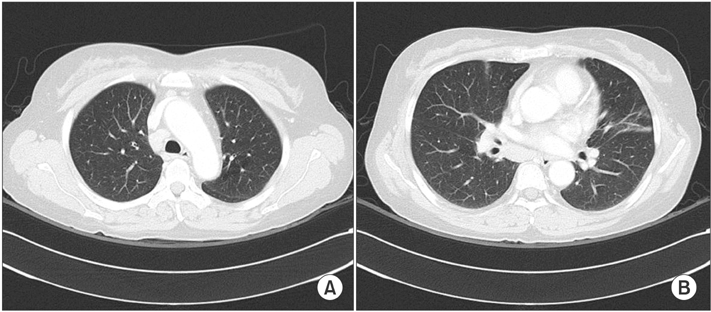
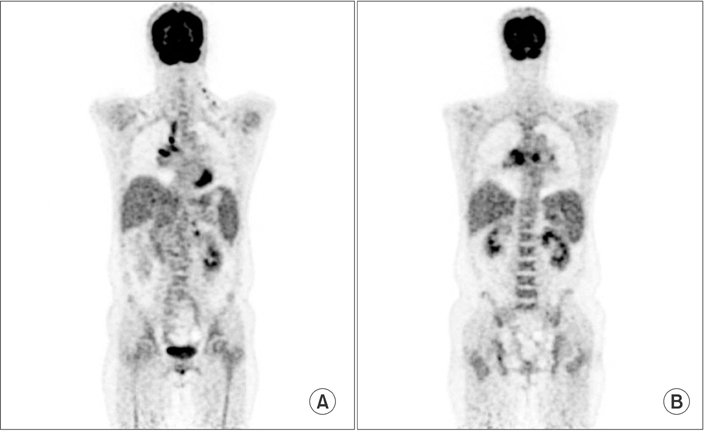
- Lymphomatoid granulomatosis (LYG) is an angiocentric and angiodestructive neoplastic proliferation of B and T lymphocytes commonly involving the lungs. Epstein-Barr virus is commonly detected in lesional cells. We report a case of a 54-year-old female with underlying monoclonal gammopathy of unknown significance who presented with a 4 week history of dyspnea and cough. Computed tomography scan of the chest showed multiple lung nodules as well as endobronchial narrowing causing atelectasis at the left upper lobe. Bronchoscopic findings revealed obstruction at the lingula segment due to endobronchial mass as a rare presentation. Bronchoscopic biopsy was diagnosed with LYG grade 1. After treatment, the endobronchial mass and lung lesions were completely resolved. However, the patient eventually evolved to malignant lymphoma after 1 year.
Keyword
MeSH Terms
Figure
Reference
-
1. Roschewski M, Wilson WH. Lymphomatoid granulomatosis. Cancer J. 2012; 18:469–474.2. Cadranel J, Wislez M, Antoine M. Primary pulmonary lymphoma. Eur Respir J. 2002; 20:750–762.3. Wilson WH, Kingma DW, Raffeld M, Wittes RE, Jaffe ES. Association of lymphomatoid granulomatosis with Epstein-Barr viral infection of B lymphocytes and response to interferonalpha 2b. Blood. 1996; 87:4531–4537.4. Fassas A, Jagannath S, Desikan KR, Shah HR, Shaver R, Waldron J, et al. Lymphomatoid granulomatosis following autologous stem cell transplantation. Bone Marrow Transplant. 1999; 23:79–81.5. Katzenstein AL, Doxtader E, Narendra S. Lymphomatoid granulomatosis: insights gained over 4 decades. Am J Surg Pathol. 2010; 34:e35–e48.6. Hare SS, Souza CA, Bain G, Seely JM, Frcpc , Gomes MM, et al. The radiological spectrum of pulmonary lymphoproliferative disease. Br J Radiol. 2012; 85:848–864.7. Mohyuddin GR, Sultan F, Khaleeq G. A rare presentation of a rare disease: pulmonary lymphomatoid granulomatosis. Case Rep Pulmonol. 2012; 2012:371490.8. Tagliavini E, Rossi G, Valli R, Zanelli M, Cadioli A, Mengoli MC, et al. Lymphomatoid granulomatosis: a practical review for pathologists dealing with this rare pulmonary lymphoproliferative process. Pathologica. 2013; 105:111–116.9. Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011; 117:5019–5032.10. Jaffe ES, Wilson WH. Lymphomatoid granulomatosis: pathogenesis, pathology and clinical implications. Cancer Surv. 1997; 30:233–248.11. Kwon OJ, Han SK, Shim YS, Kim KY, Han YC, Kim JH, et al. Lymphomatoid granulomatosis: a case report with review of literature. Korean J Intern Med. 1985; 29:124–130.12. Jung HK, Cheon SH, Lee SN, Kim SS. A case of pulomonary lymphomatold granulomatosis. Korean J Med. 1997; 52:247–252.13. Kim EH, Jang HJ, Rhee KH, Han EM, Hugh J, Kim SD, et al. A case of lymphomatoid granulomatosis refractory to various antilymphoma treatment including high dose therapy. Korean J Med. 2007; 72:S332–S337.