Tuberc Respir Dis.
2009 Aug;67(2):145-147.
A Case of Anaphylaxis after the Treatment with Etoposide in a Patient with Small Cell Lung Cancer
- Affiliations
-
- 1Department of Internal Medicine, Division of Pulmonary and Critical Care Medicine, Chonnam National University Medical School, Gwangju, Korea. cyberkks@chonnam.ac.kr
- 2Department of Internal Medicine, Division of Allergy, Asthma and Clinical Immunology, Chonnam National University Medical School, Gwangju, Korea.
Abstract
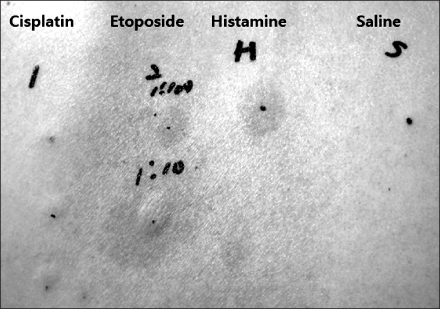
- Etoposide is a semi-synthetic derivative of podophyllotoxin that is effective against many cancers including small cell lung cancer. We report a case of etoposide-induced anaphylaxis in a 51-year-old woman who tolerated etoposide during her first cycle chemotherapy regimen. During the second cycle, she complained of generalized urticaria and dyspnea 5 minutes after being infused with etoposide. She recovered completely with antihistamine, corticosteroid and fluid replacement. The intradermal skin test with etoposide showed a clear positive immediate reaction. This case suggests that etoposide can induce IgE-mediated anaphylaxis.
Keyword
MeSH Terms
Figure
Reference
-
1. Weiss RB. Perry MC, editor. Chaptor 35. Hypersensitivity reactions. The chemotherapy source book. 2001. 3rd ed. Baltimore: Williams & Wilkins, Inc.;436–452.2. Sandler AB. Chemotherapy for small cell lung cancer. Semin Oncol. 2003. 30:9–25.3. Collier K, Schink C, Young AM, How K, Seckl M, Savage P. Successful treatment with etoposide phosphate in patients with previous etoposide hypersensitivity. J Oncol Pharm Pract. 2008. 14:51–55.4. Siderov J, Prasad P, De Boer R, Desai J. Safe administration of etoposide phosphate after hypersensitivity reaction to intravenous etoposide. Br J Cancer. 2002. 86:12–13.5. Bernstein BJ, Troner MB. Successful rechallenge with etoposide phosphate after an acute hypersensitivity reaction to etoposide. Pharmacotherapy. 1999. 19:989–991.6. Hoetelmans RM, Schornagel JH, Ten Bokkel Huinink WW, Beijnen JH. Hypersensitivity reactions to etoposide. Ann Pharmacother. 1996. 30:367–371.7. Kasperek C, Black CD. Two cases of suspected immunologic-based hypersensitivity reactions to etoposide therapy. Ann Pharmacother. 1992. 26:1227–1230.8. Taguchi A, Takeshita S, Machida R, Hori Y, Aida K, Furuya U, et al. Anaphylaxia induced by etoposide: a case report. Gan To Kagaku Ryoho. 2003. 30:1187–1189.9. Hudson MM, Weinstein HJ, Donaldson SS, Greenwald C, Kun L, Tarbell NJ, et al. Acute hypersensitivity reactions to etoposide in a VEPA regimen for Hodgkin's disease. J Clin Oncol. 1993. 11:1080–1084.10. Eschalier A, Lavarenne J, Burtin C, Renoux M, Chapuy E, Rodriguez M. Study of histamine release induced by acute administration of antitumor agents in dogs. Cancer Chemother Pharmacol. 1988. 21:246–250.11. O'Dwyer PJ, King SA, Fortner CL, Leyland-Jones B. Hypersensitivity reactions to teniposide (VM-26): an analysis. J Clin Oncol. 1986. 4:1262–1269.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment of Small Cell Lung Cancer
- Chemotherapy for Small Cell Lung Cancer
- Etoposide and cisplatin combination chemotherapy in extensive stage small cell lung cancer
- Alterating combination chemotherapy of cyclophosphamide, adriamycin, and vincristine(CAV) with etoposide and cisplatin(EP) in small cell lung cancer
- Phase II trial of 5-FU, etoposide, cisplatin (FEP) combination chemotherapy in unresectable non-small cell lung cancer