Tuberc Respir Dis.
2009 Feb;66(2):110-115.
Comparison of Forcep-biopsy and Cryo-biopsy by a Flexible Bronchoscopy
- Affiliations
-
- 1Department of Internal Medicine, Kosin University College of Medicine, Busan, Korea. oaks70@hanmail.net
- 2Department of Pathology, Kosin University College of Medicine, Busan, Korea.
Abstract
-
BACKGROUND: A forceps-biopsy is performed to acquire tissue from patients with an endobronchial carcinoma using a flexible bronchoscope. Recently, a cryo-biopsy has also been used to acquire tissue samples. Cryo-biopsy is the diagnostic application of extreme cold for the local destruction of abnormal living tissue. This technique is safe, with no radiation danger, no risk of electrical accidents, and a little risk of bleeding. This study compared a forceps-biopsy with a cryo-biopsy using a flexible bronchoscope, and examined the chemosensitivity and level of VEGF (vascular endothelial growth factor) in the specimens obtained from the cryo-biopsy.
METHODS
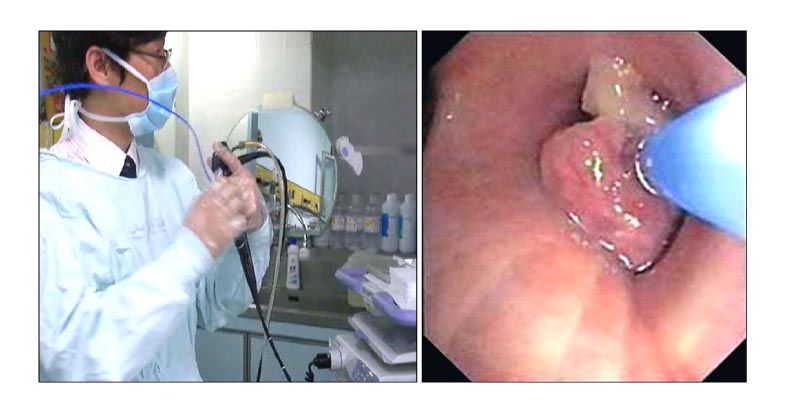
We present a prospective study of 30 consecutive patients who underwent a forceps-biopsy between January 2007 and October 2007 with a mean age of 62.1 years and a male:female ratio of 5 : 1. A flexible bronchoscope was inserted to the area of the abnormal lesions, and a cryo-probe was then applied through the working channel of the flexible bronchoscope. A temperature of approximately -80 was delivered to the tumor site for 8 seconds. The cryo-biopsy was performed after destroying the tumor mass.
RESULTS
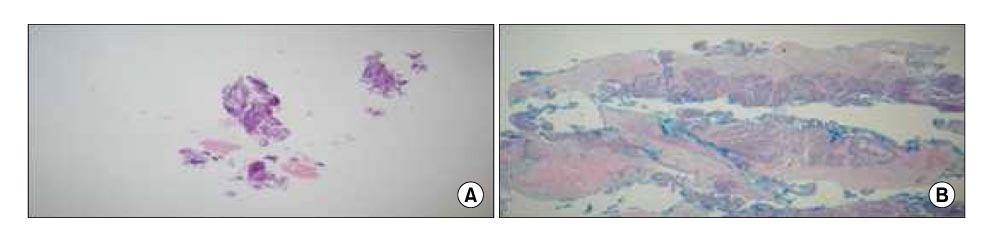
The mean size of the tissue from the forceps-biopsy and cryo-biopsy were 2.0+/-1.2 mm and 6.0+/-3.0 mm. A chemosensitivity test was performed on 5 specimens obtained using cryo-biopsy and the level of VEGF was examined in 2 specimens obtained from a cryo-biopsy. There were no side effects in either group.
CONCLUSION
Cryo-biopsy using a flexible bronchoscope is a safe and effective technique for acquiring tissue samples.
Keyword
MeSH Terms
Figure
Reference
-
1. An annual report of the cause of death in Korea. 2006. Daejeon, Korea: The National Statistical Office, Korea.2. Hattori S, Matsuda M, Komatsu T. Differentiation of lung cancer cells. Nippon Rinsho. 1966. 24:1824–1830.3. Hess FG Jr, McDowell EM, Trump BF. Pulmonary cytology: current status of cytologic typing of respiratory tract tumors. Am J Pathol. 1981. 103:323–333.4. Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008. 358:2039–2049.5. Johnston WW, Szpak CA, Thor A, Simpson JF, Schlom J. Applications of immunocytochemistry to clinical cytology. Cancer Invest. 1987. 5:593–611.6. Campling BG, Pym J, Baker HM, Cole SP, Lam YM. Chemosensitivity testing of small cell lung cancer using the MTT assay. Br J Cancer. 1991. 63:75–83.7. Ruckdeschel JC, Carney DN, Oie HK, Russell EK, Gazdar AF. In vitro chemosensitivity of human lung cancer cell lines. Cancer Treat Rep. 1987. 71:697–704.8. Fujii Y, Ohno N, Li Z, Terada N, Baba T, Ohno S. Morphological and histochemical analyses of living mouse livers by new 'cryobiopsy' technique. J Electron Microsc (Tokyo). 2006. 55:113–122.9. Ohno N, Terada N, Bai Y, Saitoh S, Nakazawa T, Nakamura N, et al. Application of cryobiopsy to morphological and immunohistochemical analyses of xenografted human lung cancer tissues and functional blood vessels. Cancer. 2008. 113:1068–1079.10. Hoffman RM. In vitro assays for chemotherapy sensitivity. Crit Rev Oncol Hematol. 1993. 15:99–111.11. Kang HJ, Ko CD, Yoon HS, Kim MB, Ahn SH. The reliability of histoculture drug response assay (HDRA) in chemosensitivity tests for breast cancer. Cancer Res Treat. 2001. 33:392–397.12. Neel HB 3rd, Farrell KH, DeSanto LW, Payne WS, Sanderson DR. Cryosurgery of respiratory structures. I. Cryonecrosis of trachea and bronchus. Laryngoscope. 1973. 83:1062–1071.13. Homasson JP, Renault P, Angebault M, Bonniot JP, Bell NJ. Bronchoscopic cryotherapy for airway strictures caused by tumors. Chest. 1986. 90:159–164.14. Mazur P. Cryobiology: the freezing of biological systems. Science. 1970. 168:939–949.15. Rubinsky B, Lee CY, Bastacky J, Onik G. The process of freezing and the mechanism of damage during hepatic cryosurgery. Cryobiology. 1990. 27:85–97.16. Sheski FD, Mathur PN. Cryotherapy, electrocautery, and brachytherapy. Clin Chest Med. 1999. 20:123–138.17. Park JY, Kim YS, Bang S, Hyung WJ, Noh SH, Choi SH, et al. ATP-based chemotherapy response assay in patients with unresectable gastric cancer. Oncology. 2007. 73:439–440.18. Ohie S, Udagawa Y, Aoki D, Nozawa S. Histoculture drug response assay to monitor chemoresponse. Methods Mol Med. 2005. 110:79–86.19. Chan AL, Yoneda KY, Allen RP, Albertson TE. Advances in the management of endobronchial lung malignancies. Curr Opin Pulm Med. 2003. 9:301–308.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fluoroscopy-Guided Transnasal Biopsy of Nasopharyngeal Carcinoma Using a Flexible Bronchoscopic Biopsy Forcep
- A Case of Capillary Hemangioma of Lingular Segmental Bronchus in Adult
- Comparison of Endoscopic Forcep Biopsy and the Histopathologic Diagnosis after Endoscopic Submucosal Dissection
- Diagnosis of Suspected Active Pulmonary Tuberculosis by Flexible Fiberoptic Bronchoscopy
- The Pathological Differences of Colorectal Polyps Examined between the Use of a Forcep Biopsy and Endoscopic Resection