Korean J Urol.
2013 Jul;54(7):433-436.
External Validation of the Cancer of the Prostate Risk Assessment-S Score in Koreans Undergoing Radical Prostatectomy
- Affiliations
-
- 1Department of Urology, Gangneung Asan Hospital, University of Ulsan College of Medicine, Gangneung, Korea. jypark@gnah.co.kr
Abstract
- PURPOSE
To evaluate the validity of the University of California San Francisco Cancer of the Prostate Risk Assessment-S score (CAPRA-S score), a biochemical indicator of recurrent prostate cancer that uses histopathologic data, in Korean prostate cancer patients.
MATERIALS AND METHODS
A total of 203 prostate cancer patients who underwent radical prostatectomy between February 1997 and November 2010 were observed for longer than 6 months. The CAPRA-S score of 134 patients for whom records were available for preoperative prostate-specific antigen (PSA), pathologic specimen Gleason score, surgical margin, seminal vesicle invasion, extracapsular extension, and lymph node invasion were calculated. Biochemical recurrence was defined as repetitive measurement of PSA > or =0.2 ng/mL at least 6 months after surgery with at least a 4-week interval. The Cox proportional hazard model and Kaplan-Meier analysis were used for the statistical testing.
RESULTS
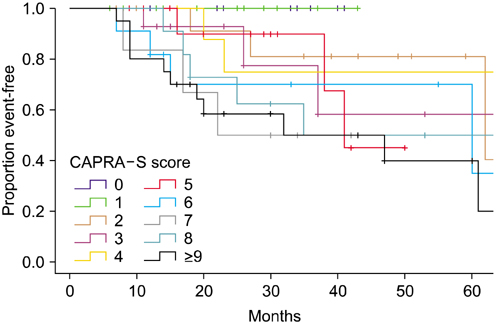
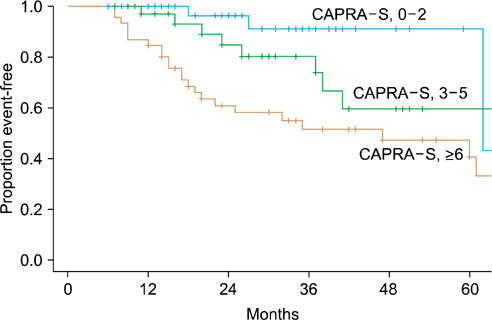
The CAPRA-S scores were divided into nine groups. The 5-year disease-free survival rate was reduced as the CAPRA-S score increased compared with the group with a CAPRA-S score of 0-1. The CAPRA-S score in this study was more sensitive to biochemical recurrence than was the CAPRA score conducted at this institution (CAPRA-S concordance index, 0.776; CAPRA concordance index, 0.728).
CONCLUSIONS
The CAPRA-S score is judged to be a useful tool for predicting the disease-free survival rate of Korean prostate cancer patients and is thought to assist in establishing postoperative management.
MeSH Terms
Figure
Reference
-
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010. 60:277–300.2. Cooperberg MR, Pasta DJ, Elkin EP, Litwin MS, Latini DM, Du Chane J, et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. 2005. 173:1938–1942.3. Zhao KH, Hernandez DJ, Han M, Humphreys EB, Mangold LA, Partin AW. External validation of University of California, San Francisco, Cancer of the Prostate Risk Assessment score. Urology. 2008. 72:396–400.4. Lughezzani G, Budaus L, Isbarn H, Sun M, Perrotte P, Haese A, et al. Head-to-head comparison of the three most commonly used preoperative models for prediction of biochemical recurrence after radical prostatectomy. Eur Urol. 2010. 57:562–568.5. Cooperberg MR, Freedland SJ, Pasta DJ, Elkin EP, Presti JC Jr, Amling CL, et al. Multiinstitutional validation of the UCSF cancer of the prostate risk assessment for prediction of recurrence after radical prostatectomy. Cancer. 2006. 107:2384–2391.6. May M, Knoll N, Siegsmund M, Fahlenkamp D, Vogler H, Hoschke B, et al. Validity of the CAPRA score to predict biochemical recurrence-free survival after radical prostatectomy. Results from a european multicenter survey of 1,296 patients. J Urol. 2007. 178:1957–1962.7. Shariat SF, Karakiewicz PI, Roehrborn CG, Kattan MW. An updated catalog of prostate cancer predictive tools. Cancer. 2008. 113:3075–3099.8. Cooperberg MR, Broering JM, Carroll PR. Risk assessment for prostate cancer metastasis and mortality at the time of diagnosis. J Natl Cancer Inst. 2009. 101:878–887.9. Cooperberg MR, Hilton JF, Carroll PR. The CAPRA-S score: a straightforward tool for improved prediction of outcomes after radical prostatectomy. Cancer. 2011. 117:5039–5046.10. Vickers A. Prediction models in urology: are they any good, and how would we know anyway? Eur Urol. 2010. 57:571–573.11. Penson DF, Chan JM. Urologic Diseases in America Project. Prostate cancer. J Urol. 2007. 177:2020–2029.12. Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005. 294:433–439.13. Bauer JJ, Connelly RR, Sesterhenn IA, Bettencourt MC, McLeod DG, Srivastava S, et al. Biostatistical modeling using traditional variables and genetic biomarkers for predicting the risk of prostate carcinoma recurrence after radical prostatectomy. Cancer. 1997. 79:952–962.14. Kattan MW, Wheeler TM, Scardino PT. Postoperative nomogram for disease recurrence after radical prostatectomy for prostate cancer. J Clin Oncol. 1999. 17:1499–1507.15. Stephenson AJ, Scardino PT, Eastham JA, Bianco FJ Jr, Dotan ZA, Fearn PA, et al. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst. 2006. 98:715–717.16. Messing EM, Manola J, Yao J, Kiernan M, Crawford D, Wilding G, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006. 7:472–479.17. Thompson IM, Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009. 181:956–962.18. Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003. 169:517–523.19. Burkhard FC, Bader P, Schneider E, Markwalder R, Studer UE. Reliability of preoperative values to determine the need for lymphadenectomy in patients with prostate cancer and meticulous lymph node dissection. Eur Urol. 2002. 42:84–90.20. Touijer K, Rabbani F, Otero JR, Secin FP, Eastham JA, Scardino PT, et al. Standard versus limited pelvic lymph node dissection for prostate cancer in patients with a predicted probability of nodal metastasis greater than 1%. J Urol. 2007. 178:120–124.21. Mattei A, Fuechsel FG, Bhatta Dhar N, Warncke SH, Thalmann GN, Krause T, et al. The template of the primary lymphatic landing sites of the prostate should be revisited: results of a multimodality mapping study. Eur Urol. 2008. 53:118–125.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Radical Prostatectomy
- A Case of No Residual Cancer in Radical Prostatectomy Specimens Despite Biopsy-proven Prostate Cancer
- Role of Radical Prostatectomy for High-Risk Prostate Cancer
- Predictors of Gleason Score Upgrading after Radical Prostatectomy in Low-Risk Prostate Cancer
- Cancer of the Prostate Risk Assessment (CAPRA) Preoperative Score Versus Postoperative Score (CAPRA-S): Ability to Predict Cancer Progression and Decision-Making Regarding Adjuvant Therapy after Radical Prostatectomy