Korean J Urol.
2012 Mar;53(3):149-153.
Clinical Significance of Free-to-Total Prostate-Specific Antigen (PSA) Ratio in Advanced Prostate Cancer Patients with PSA Less than 0.1 ng/ml after Hormone Treatment
- Affiliations
-
- 1Department of Urology, Yonsei University Wonju College of Medicine, Wonju, Korea. chc7174@yonsei.ac.kr
Abstract
- PURPOSE
We analyzed the pattern of change in the free-to-total prostate-specific antigen (f/t PSA) ratio and the progression to castration-resistant prostate cancer (CRPC) in patients with advanced prostate cancer who received hormone treatment and whose PSA nadir was below 0.1 ng/ml.
MATERIALS AND METHODS
We retrospectively analyzed the medical records of 52 patients with advanced prostate cancer. All patients were treated with maximum androgen blockade (gonadotrophin-releasing hormone agonist and anti-androgen agents). The patients were divided into two groups: those with a nadir f/t PSA ratio above 60% and those with a nadir f/t PSA ratio of 60% or below. Age, initial PSA, clinical stage, lymph node metastasis, bone metastasis, and follow-up data, including PSA, free PSA, and f/t PSA ratio, were collected. The Mann-Whitney U-test, Fisher exact test, chi-square test, Kaplan-Meier survival analysis, and log rank test were used.
RESULTS
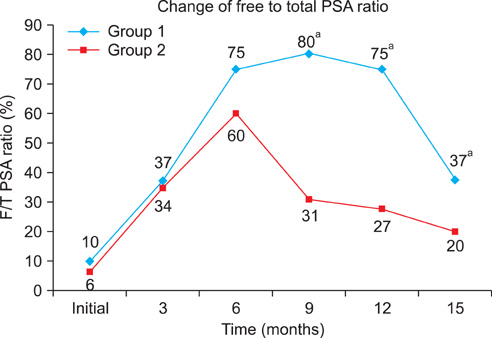
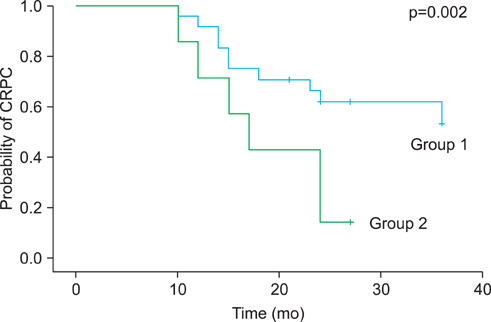
There were 24 patients in the group with a nadir f/t PSA ratio above 60% and 28 patients in the group with a nadir f/t PSA ratio of 60% or below. After hormone therapy, the median f/t PSA ratio in each group increased from 37% and 34% at 3 months to 75% and 60% at 6 months, respectively. At 9 months, however, the f/t PSA ratio increased to 80% in the group with a nadir f/t PSA ratio above 60%, whereas it decreased to 31% in the group with a nadir f/t PSA ratio of 60% or below. From 9 to 15 months, the f/t PSA ratio showed a tendency to decrease (75 to 37% and 27 to 20%, respectively). The progression to CRPC was significantly different between the two groups (10 vs. 24).
CONCLUSIONS
Progression to CRPC was significantly higher in the group with a lower f/t PSA ratio. Additionally, the pattern of change in the f/t PSA ratio was significantly different after 9 months. Collectively, the f/t PSA ratio can be used as an additional marker for prognosis of hormone treatment.
MeSH Terms
Figure
Reference
-
1. American Cancer Society. Cancer facts and figures 2005. 2005. Atlanta: American Cancer Society.2. Ministry for Heath, Welfare and Family Affairs. Annual report of cancer incidence (2006) and survival (1993-2006) in Korea. 2009. Seoul: Ministry for Heath, Welfare and Family Affairs.3. Derweesh IH, Kupelian PA, Zippe C, Levin HS, Brainard J, Magi-Galluzzi C, et al. Continuing trends in pathological stage migration in radical prostatectomy specimens. Urol Oncol. 2004. 22:300–306.4. Mettlin C, Murphy GP, Babaian RJ, Chesley A, Kane RA, Littrup PJ, et al. The results of a five-year early prostate cancer detection intervention: investigators of the american cancer society national prostate cancer detection project. Cancer. 1996. 77:150–159.5. Park SC, Choi HY, Kim CS, Hong SJ, Kim WJ, Lee SE, et al. Predictive variables of the progression to androgen independent prostate cancer after combined androgen blockade. Korean J Urol. 2007. 48:408–415.6. Mottet N, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2011. 59:572–583.7. Heidenreich A, Aus G, Bolla M, Joniau S, Matveev VB, Schmid HP, et al. EAU guidelines on prostate cancer. Eur Urol. 2008. 53:68–80.8. Lam JS, Leppert JT, Vemulapalli SN, Shvarts O, Belldegrun AS. Secondary hormonal therapy for advanced prostate cancer. J Urol. 2006. 175:27–34.9. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010. 17:1471–1474.10. Klotz L. Maximal androgen blockade for advanced prostate cancer. Best Pract Res Clin Endocrinol Metab. 2008. 22:331–340.11. Kwak C, Jeong SJ, Park MS, Lee E, Lee SE. Prognostic significance of the nadir prostate specific antigen level after hormone therapy for prostate cancer. J Urol. 2002. 168:995–1000.12. Stewart AJ, Scher HI, Chen MH, McLeod DG, Carroll PR, Moul JW, et al. Prostate-specific antigen nadir and cancer specific mortality following hormonal therapy for prostate specific antigen failure. J Clin Oncol. 2005. 23:6556–6560.13. Cooperberg MR, Lubeck DP, Mehta SS, Carroll PR. Time trends in clinical risk stratification for prostate cancer: implications for outcomes (data from CaPSURE). J Urol. 2003. 170(6 Pt 2):S21–S27.14. Kaliks RA, Del Giglio A. Management of advanced prostate cancer. Rev Assoc Med Bras. 2008. 54:178–182.15. van den Ouden D, Schröder FH. Management of locally advanced prostate cancer. 1. Staging, natural history, and results of radical surgery. World J Urol. 2000. 18:194–203.16. Schröder FH, van den Ouden D. Management of locally advanced prostate cancer 2. Radiotherapy, neoadjuvanct endocrine treatment, update 1997-1999. World J Urol. 2000. 18:204–215.17. Rosenthal SA, Sandler HM. Treatment strategies for high-risk locally advanced prostate cancer. Nat Rev Urol. 2010. 7:31–38.18. Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part I: Screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011. 59:61–71.19. Heidenreich A. Current approach to androgen deprivation therapy in patients with advanced prostate cancer. Eur Urol Suppl. 2010. 9:776–781.20. Huang SP, Bao BY, Wu MT, Choueiri TK, Goggins WB, Huang CY, et al. Impact of prostate-specific antigen (PSA) nadir and time to PSA nadir on disease progression in prostate cancer treated with androgen-deprivation therapy. Prostate. 2011. 71:1189–1197.21. Collette L, Burzykowski T, Schröder FH. Prostate-specific antigen (PSA) alone is not an appropriate surrogate marker of long-term therapeutic benefit in prostate cancer trials. Eur J Cancer. 2006. 42:1344–1350.22. Yoon CY. Free PSA and the free PSA to total PSA ratio as predictor of response to hormone treatment for metastatic prostate cancer. Korean J Urol. 2006. 47:362–367.23. Tanaka M, Murakami S, Suzuki N, Shimazaki J. Change in the ratio of free-to-total prostate-specific antigen during progression of advanced prostate cancer. Int J Urol. 2000. 7:83–87.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Free/Total PSA in the Differential Diagnosis of the Prostate Cancer and Benign Prostatic Hyperplasia
- Association of Prostate Specific Antigen (PSA) Velocity with Age and Initial PSA in Healthy Korean Men
- Prostate specific antigen as a tumor marker for adenocarcinoma of the prostate
- Age Specific Free PSA to Total PSA Ratio in Normal Korean Men
- Free PSA and the Free PSA to Total PSA Ratio as a Predictor of Response to Hormone Treatment for Metastatic Prostate Cancer