Obstet Gynecol Sci.
2015 Mar;58(2):162-170. 10.5468/ogs.2015.58.2.162.
Therapy of heavy menstrual bleeding in Korea: Subanalysis and results from a multinational clinical trial in the Asian region investigating the levonorgestrel-releasing intrauterine system versus conventional therapy
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. dr222@yuhs.ac
- 2Department of Gynecology and Obstetrics, Peking Union Medical College Hospital, Beijing, China.
- 3Department of Gynecology and Obstetrics, Surgimed Hospital, Lahore, Pakistan.
- 4Bayer HealthCare, Asia-Pacific Women's Healthcare, Singapore.
- 5Bayer HealthCare, Global Non-Intervention Studies, Germany.
- 6Bayer HealthCare, Global Medical Affairs Women's Healthcare, Berlin, Germany.
- 7Bayer HealthCare, Medical Affairs, Korea.
- KMID: 2314041
- DOI: http://doi.org/10.5468/ogs.2015.58.2.162
Abstract
OBJECTIVE
To compare real-life clinical outcomes with the levonorgestrel-releasing intrauterine system (LNG-IUS) and conventional medical therapies (CMTs), including combined oral contraceptives and oral progestins in the treatment of idiopathic heavy menstrual bleeding (HMB) in South Korea.
METHODS
This prospective, observational cohort study recruited a total of 647 women aged 18 to 45 years, diagnosed with HMB from 8 countries in Asia, including 209 women from South Korea (LNG-IUS, 169; CMTs, 40), who were followed up to one year. The primary outcome was cumulative continuation rate (still treated with LNG-IUS and CMTs) at 12 months. Secondary outcomes included bleeding pattern, assessment of the treatment efficacy by treating physician and safety profile.
RESULTS
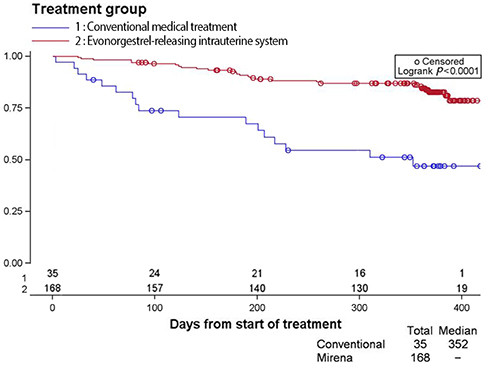
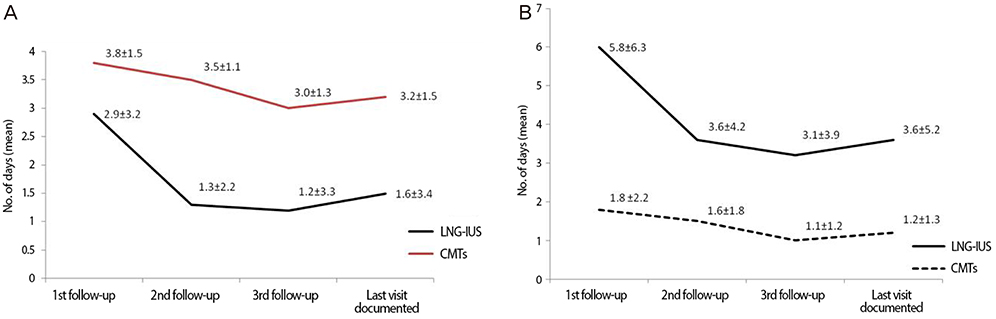
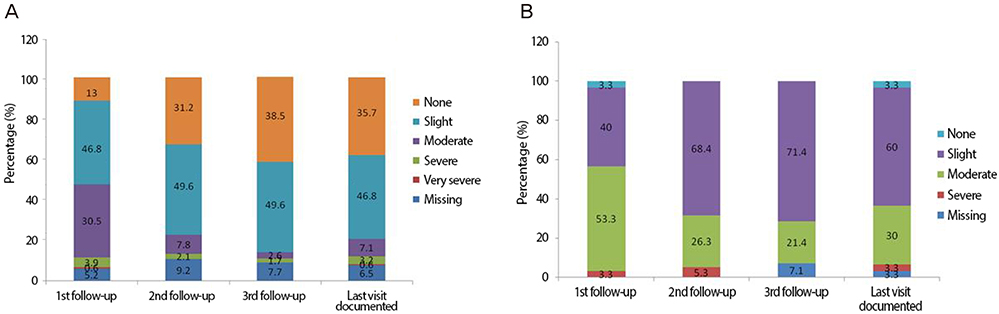
The continuation rate at 12 months was significantly higher with the LNG-IUS than CMTs (85.1% vs. 48.5%, respectively; P<0.0001). The 51.5% of CMTs patients discontinued treatment and 18.8% of LNG-IUS patients discontinued treatment. The most common reasons for discontinuation for CMTs were switching to another treatment and personal reasons. When compared to CMTs, the LNG-IUS offered better reduction in subjectively assessed menstrual blood loss and the number of bleeding days, tolerability and with better efficacy in HMB, as assessed by physician's final evaluation.
CONCLUSION
This study provides novel information on the real-life treatment patterns of HMB in South Korea. The efficacy of CMTs was inferior compared to the LNG-IUS in the clinical outcomes measured in this study. Due to the better compliance with LNG-IUS, the cumulative continuation rate is higher than CMTs. We conclude that the LNG-IUS should be used as the first-line treatment for HMB in Korean women, in line with international guidelines.
MeSH Terms
Figure
Reference
-
1. National Institutes for Health and Care Excellence. Heavy menstrual bleeding: NICE guidelines CG44 [Internet]. London: National Institutes for Health and Care Excellence;c2012. cited 2012 Jan 4. Available from: http://www.nice.org.uk/nicemedia/pdf/CG44NICEGuideline.pdf.2. Oehler MK, Rees MC. Menorrhagia: an update. Acta Obstet Gynecol Scand. 2003; 82:405–422.3. El-Hemaidi I, Gharaibeh A, Shehata H. Menorrhagia and bleeding disorders. Curr Opin Obstet Gynecol. 2007; 19:513–520.4. Lopes JE Jr, Sherer E. Managing menorrhagia: evaluating and treating heavy menstrual bleeding. Adv NPs PAs. 2010; 1:21–24.5. Royal College of Obstetricians and Gynaecologists. The initial management of menorrhagia: evidence-based clinical guidelines no.1 [Internet]. London: Royal College of Obstetricians and Gynaecologists;c1998. cited 2012 Jan 20. Available from: http://www.rcog.org.uk.6. Duckitt K, McCully K. Menorrhagia. Clin Evid. 2003; (10):2151–2169.7. Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefitrisk review. Drugs. 2012; 72:193–215.8. Tang GW, Lo SS. Levonorgestrel intrauterine device in the treatment of menorrhagia in Chinese women: efficacy versus acceptability. Contraception. 1995; 51:231–235.9. Xiao B, Wu SC, Chong J, Zeng T, Han LH, Luukkainen T. Therapeutic effects of the levonorgestrel-releasing intrauterine system in the treatment of idiopathic menorrhagia. Fertil Steril. 2003; 79:963–969.10. Shaaban MM, Zakherah MS, El-Nashar SA, Sayed GH. Levonorgestrel-releasing intrauterine system compared to low dose combined oral contraceptive pills for idiopathic menorrhagia: a randomized clinical trial. Contraception. 2011; 83:48–54.11. Kaunitz AM, Bissonnette F, Monteiro I, Lukkari-Lax E, Muysers C, Jensen JT. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010; 116:625–632.12. Robinson R, China S, Bunkheila A, Powell M. Mirena intrauterine system in the treatment of menstrual disorders: a survey of UK patients' experience, acceptability and satisfaction. J Obstet Gynaecol. 2008; 28:728–731.13. Ewies AA. Levonorgestrel-releasing intrauterine system: the discontinuing story. Gynecol Endocrinol. 2009; 25:668–673.14. Cooper KG, Jack SA, Parkin DE, Grant AM. Five-year follow up of women randomised to medical management or transcervical resection of the endometrium for heavy menstrual loss: clinical and quality of life outcomes. BJOG. 2001; 108:1222–1228.15. Coulter A, Bradlow J, Agass M, Martin-Bates C, Tulloch A. Outcomes of referrals to gynaecology outpatient clinics for menstrual problems: an audit of general practice records. Br J Obstet Gynaecol. 1991; 98:789–796.16. Tariq N, Ayub R, Jaffery T, Rahim F, Naseem F, Kamal M. Efficacy of levonorgestrel intrauterine system (LNG-IUS) for abnormal uterine bleeding and contraception. J Coll Physicians Surg Pak. 2011; 21:210–213.17. Yoo HJ, Lee MA, Ko YB, Yang JB, Kang BH, Lee KH. The efficacy of the levonorgestrel-releasing intrauterine system in perimenopausal women with menorrhagia or dysmenorrhea. Arch Gynecol Obstet. 2012; 285:161–166.18. Lee BS, Ling X, Asif S, Kraemer P, Hanisch JU, Inki P. Levonorgestrel-releasing intrauterine system versus conventional medical therapy for heavy menstrual bleeding in the Asia-Pacific region. Int J Gynaecol Obstet. 2013; 121:24–30.19. Shaw RW, Brickley MR, Evans L, Edwards MJ. Perceptions of women on the impact of menorrhagia on their health using multi-attribute utility assessment. Br J Obstet Gynaecol. 1998; 105:1155–1159.20. Marret H, Fauconnier A, Chabbert-Buffet N, Cravello L, Golfier F, Gondry J, et al. Clinical practice guidelines on menorrhagia: management of abnormal uterine bleeding before menopause. Eur J Obstet Gynecol Reprod Biol. 2010; 152:133–137.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Levonorgestrel-Releasing Intrauterine System Use in Perimenopausal Women
- Clinical applications of levonorgestrel-releasing intrauterine system to gynecologic diseases
- Effectiveness of Levonorgestrel Releasing Intrauterine System in Perimenopausal Women with Heavy Menstrual Bleeding: A Prospective Study at a Teaching Hospital in India
- Clinical Efficacy of Levonorgestrel-Releasing Intrauterine System (Mirena(R)) for Abnormal Uterine Bleeding
- Therapeutic efficacy of Mirena in gynecologic disease