Nutr Res Pract.
2014 Jun;8(3):272-277.
Schisandra Chinensis Baillon regulates the gene expression of phase II antioxidant/detoxifying enzymes in hepatic damage induced rats
- Affiliations
-
- 1Department of Nutritional Science and Food Management, Ewha Womans University, 52, Ewhayeodae-gil, Seodaemun-gu, Seoul 120-750, Republic of Korea. orank@ewha.ac.kr
- 2Department of Biomedical Laboratory Science, Eulji University, Seongam-si, Gyeonggi-do, 461-713, Republic of Korea.
Abstract
- BACKGROUND/OBJECTIVES
This study investigated the antioxidant activities and hepatoprotective effects of Schisandra chinensis Baillon extract (SCE) against tert-butyl hydroperoxide (t-BHP)-induced oxidative hepatic damage in rats.
MATERIALS/METHODS
Sprague-Dawley (SD) rats were pretreated with SCE (300, 600, and 1,200 mg/kg BW) or saline once daily for 14 consecutive days. On day 14, each animal, except those belonging to the normal control group, were injected with t-BHP (0.8 mmol/kg BW/i.p.), and all of the rats were sacrificed 16 h after t-BHP injection.
RESULTS
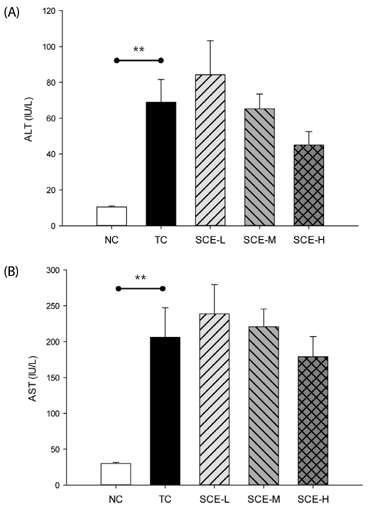
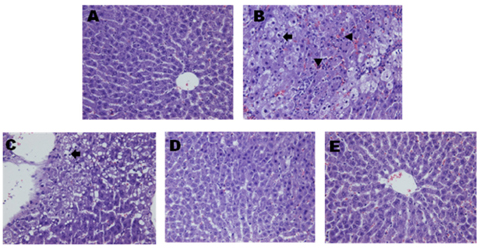
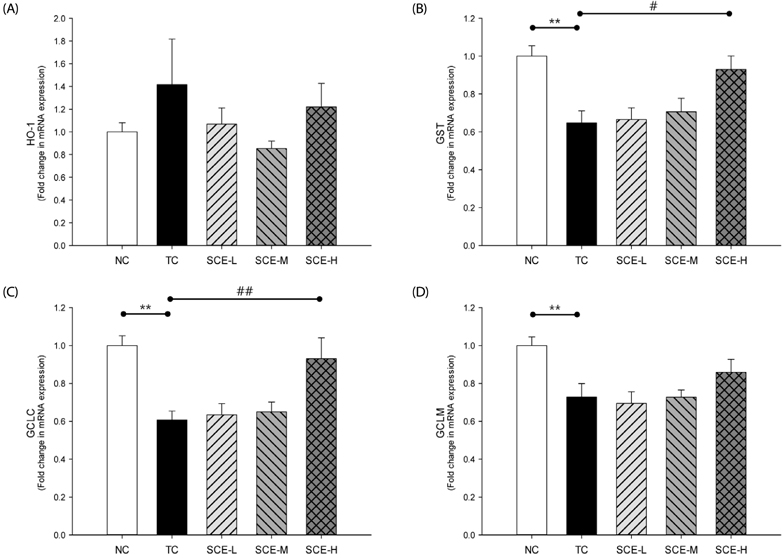
Although no significant differences in AST and ALT levels were observed among the TC and SCE groups, the high-dose SCE group showed a decreasing tendency compared to the TC group. However, erythrocyte SOD activity showed a significant increase in the low-dose SCE group compared with the TC group. On the other hand, no significant differences in hepatic total glutathione (GSH) level, glutathione reductase (GR), and glutathione peroxidase (GSH-Px) activities were observed among the TC and SCE groups. Hepatic histopathological evaluation revealed that pretreatment with SCE resulted in reduced t-BHP-induced incidence of lesions, such as neutrophil infiltration, swelling of liver cells, and necrosis. In particular, treatment with a high dose of SCE resulted in induction of phase II antioxidant/detoxifying enzyme expression, such as glutathione S-transferase (GST) and glutamate-cysteine ligase catalytic subunit (GCLC).
CONCLUSIONS
Based on these results, we conclude that SCE exerts protective effects against t-BHP induced oxidative hepatic damage through the reduction of neutrophil infiltration, swelling of liver cells, and necrosis. In addition, SCE regulates the gene expression of phase II antioxidant/detoxifying enzymes independent of hepatic antioxidant enzyme activity.
Keyword
MeSH Terms
-
Animals
Catalytic Domain
Erythrocytes
Gene Expression*
Glutamate-Cysteine Ligase
Glutathione
Glutathione Peroxidase
Glutathione Reductase
Glutathione Transferase
Hand
Incidence
Liver
Necrosis
Neutrophil Infiltration
Rats*
Rats, Sprague-Dawley
Schisandra*
tert-Butylhydroperoxide
Glutamate-Cysteine Ligase
Glutathione
Glutathione Peroxidase
Glutathione Reductase
Glutathione Transferase
tert-Butylhydroperoxide
Figure
Reference
-
1. Kris-Etherton PM, Lefevre M, Beecher GR, Gross MD, Keen CL, Etherton TD. Bioactive compounds in nutrition and health-research methodologies for establishing biological function: the antioxidant and anti-inflammatory effects of flavonoids on atherosclerosis. Annu Rev Nutr. 2004; 24:511–538.
Article2. Bandyopadhyay U, Das D, Banerjee RK. Reactive oxygen species: oxidative damage and pathogenesis. Curr Sci. 1999; 77:658–666.3. Chandra J, Samali A, Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med. 2000; 29:323–333.
Article4. Kanter M, Coskun O, Budancamanak M. Hepatoprotective effects of Nigella sativa L and Urtica dioica L on lipid peroxidation, antioxidant enzyme systems and liver enzymes in carbon tetrachloride-treated rats. World J Gastroenterol. 2005; 11:6684–6688.
Article5. Piñeiro-Carrero VM, Piñeiro EO. Liver. Pediatrics. 2004; 113:1097–1106.
Article6. Yang J, Li Y, Wang F, Wu C. Hepatoprotective effects of apple polyphenols on CCl4-induced acute liver damage in mice. J Agric Food Chem. 2010; 58:6525–6531.
Article7. Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005; 45:51–88.
Article8. Giudice A, Montella M. Activation of the Nrf2-ARE signaling pathway: a promising strategy in cancer prevention. Bioessays. 2006; 28:169–181.
Article9. Minotti G, Borrello S, Palombini G, Galeotti T. Cytochrome P-450 deficiency and resistance to t-butyl hydroperoxide of hepatoma microsomal lipid peroxidation. Biochim Biophys Acta. 1986; 876:220–225.
Article10. Sies H, Summer KH. Hydroperoxide-metabolizing systems in rat liver. Eur J Biochem. 1975; 57:503–512.
Article11. Martín C, Martínez R, Navarro R, Ruiz-Sanz JI, Lacort M, Ruiz-Larrea MB. tert-Butyl hydroperoxide-induced lipid signaling in hepatocytes: involvement of glutathione and free radicals. Biochem Pharmacol. 2001; 62:705–712.12. Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Ther. 2002; 96:67–202.
Article13. Hancke JL, Burgos RA, Ahumada F. Schisandra chinensis (Turcz.) baill. Fitoterapia. 1999; 70:451–471.
Article14. Jung CH, Hong MH, Kim JH, Lee JY, Ko SG, Cho K, Seog HM. Protective effect of a phenolic-rich fraction from Schisandra chinensis against H2O2-induced apoptosis in SH-SY5Y cells. J Pharm Pharmacol. 2007; 59:455–462.
Article15. Smejkal K, Slapetová T, Krmencík P, Kubínová R, Suchý P, Dall'Acqua S, Innocenti G, Vanco J, Kalvarová K, Dvorská M, Slanina J, Kramárová E, Muselík J, Zemlicka M. Evaluation of the antiradical activity of Schisandra chinensis lignans using different experimental models. Molecules. 2010; 15:1223–1231.
Article16. Ip SP, Ko KM. The crucial antioxidant action of schisandrin B in protecting against carbon tetrachloride hepatotoxicity in mice: a comparative study with butylated hydroxytoluene. Biochem Pharmacol. 1996; 52:1687–1693.
Article17. Teraoka R, Shimada T, Aburada M. The molecular mechanisms of the hepatoprotective effect of gomisin A against oxidative stress and inflammatory response in rats with carbon tetrachloride-induced acute liver injury. Biol Pharm Bull. 2012; 35:171–177.
Article18. Miquel J, Quintanilha AT, Weber H. CRC Handbook of Free Radicals and Antioxidants in Biomedicine. Volume II. Boca Raton (FL): CRC Press;1989.19. Johansson LH, Borg LA. A spectrophotometric method for determination of catalase activity in small tissue samples. Anal Biochem. 1988; 174:331–336.
Article20. Akerboom TP, Sies H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981; 77:373–382.21. Bellomo G, Mirabelli F, DiMonte D, Richelmi P, Thor H, Orrenius C, Orrenius S. Formation and reduction of glutathione-protein mixed disulfides during oxidative stress. A study with isolated hepatocytes and menadione (2-methyl-1,4-naphthoquinone). Biochem Pharmacol. 1987; 36:1313–1320.
Article22. Mohandas J, Marshall JJ, Duggin GG, Horvath JS, Tiller DJ. Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney. Possible implications in analgesic nephropathy. Biochem Pharmacol. 1984; 33:1801–1807.
Article23. Jentzsch AM, Bachmann H, Fürst P, Biesalski HK. Improved analysis of malondialdehyde in human body fluids. Free Radic Biol Med. 1996; 20:251–256.
Article24. Xiong Q, Hase K, Tezuka Y, Tani T, Namba T, Kadota S. Hepatoprotective activity of phenylethanoids from Cistanche deserticola. Planta Med. 1998; 64:120–125.
Article25. Vijayakumar RS, Nalini N. Efficacy of piperine, an alkaloidal constituent from Piper nigrum on erythrocyte antioxidant status in high fat diet and antithyroid drug induced hyperlipidemic rats. Cell Biochem Funct. 2006; 24:491–498.
Article26. Afonso V, Champy R, Mitrovic D, Collin P, Lomri A. Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine. 2007; 74:324–329.
Article27. Kensler TW. Chemoprevention by inducers of carcinogen detoxication enzymes. Environ Health Perspect. 1997; 105:Suppl 4. 965–970.
Article28. Prestera T, Talalay P, Alam J, Ahn YI, Lee PJ, Choi AM. Parallel induction of heme oxygenase-1 and chemoprotective phase 2 enzymes by electrophiles and antioxidants: regulation by upstream antioxidant-responsive elements (ARE). Mol Med. 1995; 1:827–837.
Article29. Ip SP, Ma CY, Che CT, Ko KM. Methylenedioxy group as determinant of schisandrin in enhancing hepatic mitochondrial glutathione in carbon tetrachloride-intoxicated mice. Biochem Pharmacol. 1997; 54:317–319.
Article30. Pan SY, Yu ZL, Dong H, Xiang CJ, Fong WF, Ko KM. Ethanol extract of fructus schisandrae decreases hepatic triglyceride level in mice fed with a high fat/cholesterol diet, with attention to acute toxicity. Evid Based Complement Alternat Med. 2011; 729412.
Article31. Ryu SN, Kim KS, Lee EB, Kang SS, Kim JS, Cheon SA, Lee BH. Acute toxicity of fruit pigment and seed oil of Schizandra chinensis in mice. J Korean Soc Int Agric. 1998; 10:37–41.32. Lee JS, Lee SW. Effect of water extract in fruits of Omija (Schizandra chinensis Baillon) on CCl4 toxicity. Korean J Diet Cult. 1990; 5:253–257.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Schisandrol A and gomisin N from Schisandra chinensis extract improve hypogonadism via anti-oxidative stress in TM3 Leydig cells
- Regulation of Inflammatory Repertoires and NF-kappaB Signal Transduction by DDB, an Active Compound from Schizandra Chinensis Baillon
- Suppressive effects of Schizandra chinensis Baillon water extract on allergy-related cytokine generation and degranulation in IgE-antigen complex-stimulated RBL-2H3 cells
- Lignans with NADPH Oxidase 2 (NOX2)-inhibitory Activity from the Fruits of Schisandra chinensis
- Protection by Chrysanthemum zawadskii extract from liver damage of mice caused by carbon tetrachloride is maybe mediated by modulation of QR activity