Korean J Urol.
2008 Sep;49(9):826-830.
Predictive Factors of the Long-term Medical Treatment Failure in Benign Prostatic Hyperplasia
- Affiliations
-
- 1Department of Urology, College of Medicine, Dongguk University, Gyongju, Korea. ksleemd@dongguk.ac.kr
- 2Department of Urology, Soonchunhyang University College of Medicine, Asan, Korea.
- 3Department of Urology, Ajou University School of Medicine, Suwon, Korea.
- 4Department of Urology, Ulsan University College of Medicine, Ulsan, Korea.
- 5Department of Urology, College of Medicine, Konkuk University, Seoul, Korea.
- 6Department of Urology, Keimyung University School of Medicine, Seoul, Korea.
- 7Department of Urology, College of Medicine, Kyungpook National University, Daegu, Korea.
- 8Department of Urology, Kosin University College of Medicine, Busan, Korea.
- 9Department of Urology, Chonnam National University Medical School, Gwangju, Korea.
- 10Department of Urology, Pochon CHA University College of Medicine, Pocheon, Korea.
- 11Department of Urology, Chonbuk National University Medical School, Jeonju, Korea.
- 12Department of Urology, Daegu Catholic University College of Medicine, Daegu, Korea.
- 13Department of Urology, Dong-A University College of Medicine, Busan, Korea.
- 14Department of Urology, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 15Department of Urology, Department of Urology, Inha University College of Medicine, Incheon, Korea.
- 16Department of Urology, College of Medicine, Hallym University, Chungcheon, Korea.
- 17Department of Urology, College of Medicine, Inje University, Busan, Korea.
- 18Department of Urology, College of Medicine, Yeungnam University, Daegu, Korea.
- 19Department of Urology, The Catholic University of Korea, Seoul, Korea.
- 20Department of Urology, School of Medicine, Gyeongsang National University, Jinju, Korea.
Abstract
-
PURPOSE: The aim of this study was to identify the clinical baseline factors that affect failure of medical treatment(and especially surgical treatment) for benign prostatic hyperplasia(BPH) in spite of long-term medication.
MATERIALS AND METHODS
802 men who were over 50 years of age with BPH were enrolled for this study. Patients were allocated to a medication group and a surgical treatment group(after having at least a 12 month duration of medication). We compared the differences between the two groups for their initial International Prostate Symptom Score(IPSS), the uroflowmetry, the prostate volume, the postvoid residual urine and the serum prostate specific antigen(PSA).
RESULTS
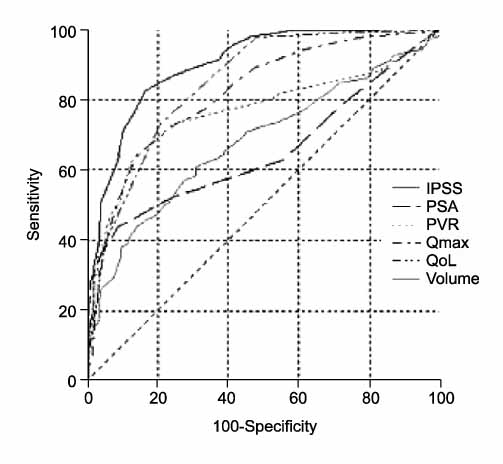
397 patients had surgical treatment following medication due to BPH progression(acute urinary retention, aggravating LUTS) and 405 patients were given maintenance medical treatment during follow-up. Statistically significant differences were found in the IPSS(23.3+/-6.6 vs. 12.7+/-8.4), the prostate volume(53.5+/-28.1ml vs. 38.3+/-12.6ml), the maximal flow rate(7.8+/-4.7ml/sec vs. 12.7+/-5.4ml/sec), the postvoid residual urine volume(92.7+/-144.4cc vs. 36.5+/-147.1cc), and the PSA(6.1+/-7.6ng/ml vs. 2.8+/- 2.8ng/ml) between the surgical and medication groups. According to the area under the curve(AUC), the IPSS, prostate volume, maximal flow rate, postvoid residual urine volume and PSA are important in descending order. According to the receiver operating characteristic(ROC) curve- based prediction of the surgical intervention, the best cutoff value for the IPSS and prostate volume were 17(area under ROC curve: 0.83) and 40ml (area under ROC curve: 0.68), respectively.
Conclusions
The results show that BPH patients with more severe IPSS (>or=17) and a larger prostate volume(>40ml) have a higher risk of surgical intervention, and this suggests that the IPSS and prostate volume may be useful predictors at the initial visit for surgical intervention.
MeSH Terms
Figure
Reference
-
1. Djavan B, Fong YK, Harik M, Milani S, Reissigl A, Chaudry A, et al. Longitudinal study of men with mild symptoms of bladder outlet obstruction treated with watchful waiting for four years. Urology. 2004. 64:1144–1148.2. McConnell JD, Bruskewitz R, Walsh P, Andriole G, Lieber M, Holtgrewe HL, et al. Finasteride Long-Term Efficacy and Safety Study Group. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. N Engl J Med. 1998. 338:557–563.3. Han KS, Hong SJ, Chung BH. Changing trends in the management of benign prostatic hyperplasia during recent 5 years. Korean J Urol. 2005. 46:458–462.4. de la Rosette JJ, Kortmann BB, Rossi C, Sonke GS, Floratos DL, Kiemeney LA. Long-term risk of re-treatment of patients using α-blockers for lower urinary tract symptoms. J Urol. 2002. 167:1734–1739.5. Djavan B, Chapple C, Milani S, Marberger M. State of the art on the efficacy and tolerability of alpha1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Urology. 2004. 64:1081–1088.6. Kirby RS, Roehrborn C, Boyle P, Bartsch G, Jardin A, Cary MM, et al. Efficacy and tolerability of doxazosin and finasteride, alone or in combination, in treatment of symptomatic benign prostatic hyperplasia: the Prospective European Doxazosin and Combination Therapy (PREDICT) trial. Urology. 2003. 61:119–126.7. Cha WH, Kim DG, Seo YJ, Lee KS. Comparison of treatment efficacy α blocker with finasteride and dutasteride in BPH. Korean J Urol. 2006. 47:Suppl. 179.8. McConnell JD, Roehrborn CG, Bautista OM, Andriole GL Jr, Dixon CM, Kusek JW, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003. 349:2387–2398.9. Roehrborn CG, Bruskewitz R, Nickel JC, McConnell JD, Saltzman B, Gittelman MC, et al. Sustained decrease in incidence of acute urinary retention and surgery with finasteride for 6 years in men with benign prostatic hyperplasia. J Urol. 2004. 171:1194–1198.10. Kim CI, Chang HS, Kim BK, Park CH. Long-term results of medical treatment in benign prostatic hyperplasia. Urology. 2006. 68:1015–1019.11. Madersbacher S, Marszalek M, Lackner J, Berger P, Schatzl G. The long-term outcome of medical therapy for BPH. Eur Urol. 2007. 51:1522–1533.12. Montorsi F, Moncada I. Safety and tolerability of treatment for BPH. Eur Urol. 2006. 5:Suppl. 1004–1012.13. Djavan B, Waldert M, Ghawidel C, Marberger M. Benign prostatic hyperplasia progression and its impact on treatment. Curr Opin Urol. 2004. 14:45–50.14. Hong SJ, Ko WJ, Kim SI, Chung BH. Identification of baseline clinical factors which predict medical treatment failure of benign prostatic hyperplasia: an observational cohort study. Eur Urol. 2003. 44:94–99.15. Roehrborn CG. Alfuzosin 10 mg once daily prevents overall clinical progression of benign prostatic hyperplasia but not acute urinary retention: results of a 2-year placebo-controlled study. BJU Int. 2006. 97:734–741.16. McNeill AS, Rizvi S, Byrne DJ. Prostate size influences the outcome after presenting with acute urinary retention. BJU Int. 2004. 94:559–562.17. Roehrborn CG, McConnell JD, Lieber M, Kaplan S, Geller J, Malek GH, et al. PLESS Study Group. Serum prostate-specific antigen concentration is a powerful predictor of acute urinary retention and need for surgery in men with clinical benign prostatic hyperplasia. Urology. 1999. 53:473–480.18. Kevin KM, Kattan MW. BPH serum markers and nomograms for selecting cnadidates for medical therapy. AUA news. 2005. 9:5–7.19. Slawin KM, Kattan MW. The use of nomograms for selecting BPH candidates for dutasteride therapy. Rev Urol. 2004. 6:Suppl 9. S40–S45.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effectiveness and Predictive Value of Responsiveness of the Transurethral Microwave Thermotherapy in the Treatment of Benign Prostatic Hyperplasia
- The influence of age and endocrine factors on the volume of benign prostatic hyperplasia
- A Prominently Large Glans penis as a Possible sign of Benign Prostatic Hyperplasia
- The Possibility of the Expert System for Benign Prostatic Hyperplasia in Symptomatic Prostatism, Using Symptom Scores, Prostatic Size and Other Factors
- Risk Factors Influencing Complications following Transurethral Prostatectomy for Benign Prostatic Hyperplasia