Kosin Med J.
2012 Dec;27(2):141-149. 10.7180/kmj.2012.27.2.141.
The Study of Animal Model of Lymphedema Using the Mouse Tail
- Affiliations
-
- 1Department of Physical Medicine and Rehabilitation, College of Medicine, Kosin University, Busan, Korea. oggum@hanmail.net
- 2Department of Pathology, College of Medicine, Kosin University, Busan, Korea.
- 3Department of Nuclear Medicine, College of Medicine, Kosin University, Busan, Korea.
- KMID: 2308523
- DOI: http://doi.org/10.7180/kmj.2012.27.2.141
Abstract
OBJECTIVES
To investigate the time course of the development of acquired and experimental lymphedema.
METHODS
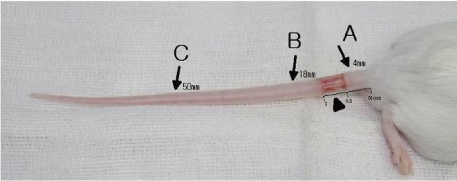
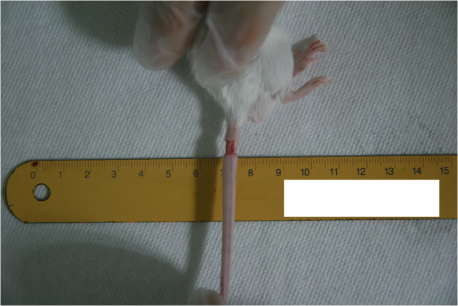
We studied an experimental model of acute post - surgical lymphedema in the tails of female hairless mice. The procedures that remove the skin and subcutaneous tissue in tails of the mice (5-10 mm from tail base) were performed, and then the murine has acquired lymphatic insufficiency. We measured volume of the tails in 2 times per week for 5 weeks, histological biopsy, and lymphoscintigraphy to assess lymphatic flow.
RESULTS
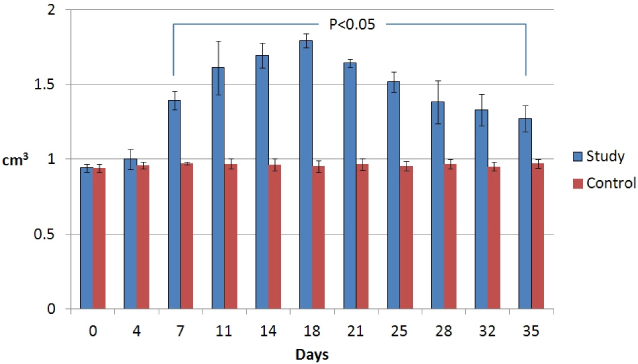
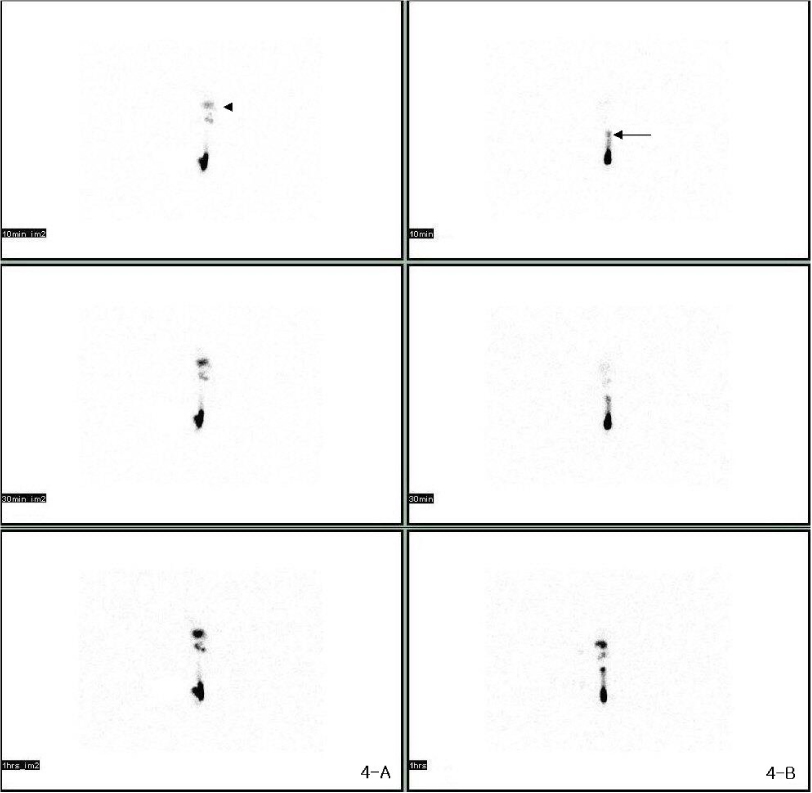
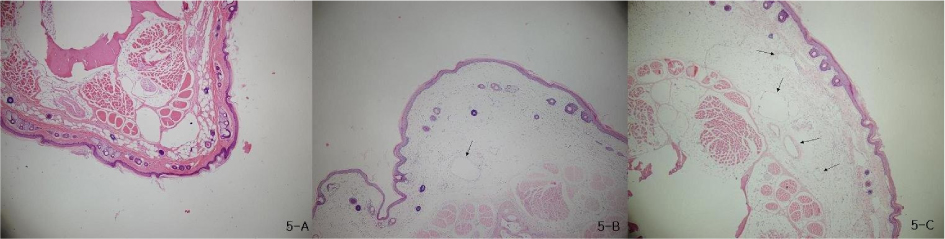
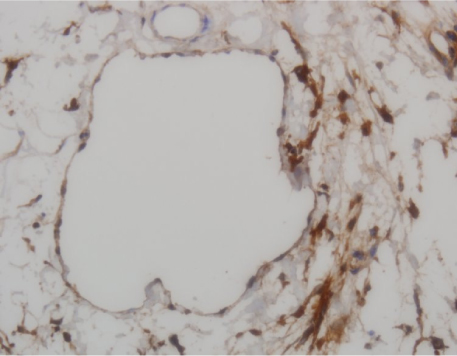
There was gradually increased volume of the tails and observed twice volume at post - surgical 18 days. In lymphoscintigraphy, we identified decreased lymphatic flow and dermal back flow in the tails. Histological biopsy showed inflammatory response that was edema and increased neutrophils in epidermis and subdermis, and lymphatic microvascular dilatation.
CONCLUSIONS
We have a mouse model of acute acquired lymphedema. This post - surgical murine tail model of lymphedema can be used to simulate an attribute of human lymphedema and provides knowledge about functional and structural alterations of lymphedema.
Keyword
MeSH Terms
Figure
Reference
-
1. Hinrichs CS, Watroba NL, Rezaishiraz H, Giese W, Hurd T, Fassl KA, Edge SB. Lymphedema secondary to postmastectomy radiation: incidence and risk factors. Ann Surg Oncol. 2004. 11:573–580.
Article2. Querci della Rovere G, Ahmad I, Singh P, Ashley S, Daniels IR, Mortimer P. An audit of the incidence of arm lymphoedema after prophylactic level I/II axillary dissection without division of the pectoralis minor muscle. Ann R Coll Surg Engl. 2003. 85:158–161.
Article3. Warren AG, Brorson H, Borud LJ, Slavin SA. Lymphedema: a comprehension review. Ann Plast Surg. 2007. 59:464–472.4. Beilhack A, Rockson SG. Immune traffic: a functional overview. Lymphat Res Biol. 2003. 1:219–234.
Article5. Modi S, Stanton AW, Mellor RH, Peters AM, Levick JR, Mortimer PS. Regional distribution of epifascial swelling and epifascial lymph drainage rate constants in breast cancer-related lymphedema. Lymphat Res Biol. 2005. 3:3–15.
Article6. Taylor R, Jayasinghe UW, Koelmeyer L, Ung O, Boyages J. Reliability and validity of arm volume measurements for assessment of lymphedema. Phys Ther. 2006. 86:205–214.
Article7. Tabibiazar R, Cheung L, Han J, Swanson J, Beihack A, An A, et al. Inflammatory manifestations of experimental lymphatic insufficiency. PLoS Med. 2006. 3:e254.
Article8. Rutkowski JM, Moya M, Johannes J, Goldman J, Swartz MA. Secondary lymphedema in the mouse tail: lymphatic hyperplasia, VEGF-C upregulation, and the protective role of MMP-9. Microvasc Res. 2006. 72:161–171.
Article9. Slavin SA, Van den Abbeele AD, Losken A, Swartz MA, Jain RK. Return of lymphatic function after flap transfer for acute lymphedema. Ann Surg. 1999. 229:421–427.
Article10. Piller NB. Macrophage and tissue changes in the developmental phases of secondary lymphoedema and during conservative therapy with benzopyrone. Arch Histol Cytol. 1990. 53:209–218.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Phototoxic reaction to amidarone as studied with the mouse tail technique and the candida albicans test
- A Case of Conjunctival and Lid Lymphedema Confirmed with Lymphoscintigraphy
- Mouse models for hepatitis B virus research
- Induction of Animal Model of Scleroderma with Repeated Injection of Bleomycin
- Management of Lymphedema