J Gynecol Oncol.
2013 Jul;24(3):280-286. 10.3802/jgo.2013.24.3.280.
The course of fatigue in patients with gynecologic and breast cancer
- Affiliations
-
- 1Department of Medical Psychology and Medical Sociology, University of Leipzig, Leipzig, Germany. andreas.hinz@medizin.uni-leipzig.de
- 2Institute of Medical Biometry, Epidemiology and Informatics, University of Mainz, Mainz, Germany.
- 3Department of Gynecology and Obstetrics, University of Leipzig, Leipzig, Germany.
- KMID: 2288536
- DOI: http://doi.org/10.3802/jgo.2013.24.3.280
Abstract
OBJECTIVE
The objective of this study is to examine the course of fatigue in female cancer patients during the first months after treatment.
METHODS
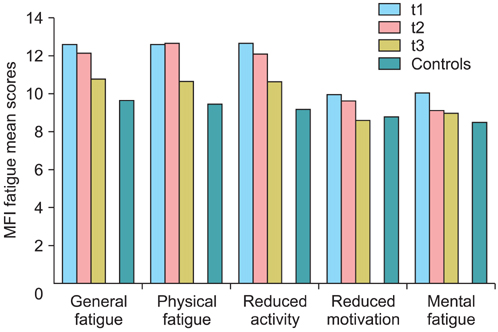
We examined a sample of 110 patients suffering from gynecological or breast cancer. Fatigue was assessed with two questionnaires, the Multidimensional Fatigue Inventory (MFI) and the fatigue scale of the quality of life questionnaire European Organisation for Research and Treatment of Cancer (EORTC QLQ-C30). Participants were tested during their stay in the hospital (t1), two weeks after discharge (t2), and three months after discharge (t3).
RESULTS
Fatigue in the patients' sample was markedly higher than the general population reference values. At t1, the effect sizes are d=0.81 (MFI) and d=1.21 (EORTC QLQ-C30 fatigue scale). Age and tumor stage had no significant influence on fatigue, but patients with a long time since diagnosis had higher fatigue levels than patients with a shorter time since diagnosis. From t1 to t3, fatigue mean scores decreased. The correlations between the t1 and the t3 fatigue scores were weak, with correlation coefficients of only about 0.30.
CONCLUSION
Though the mean scores of fatigue, averaged across all patients, decreased over the first three months, the individual courses could not be predicted from the t1 score.
MeSH Terms
Figure
Reference
-
1. Mock V, Atkinson A, Barsevick A, Cella D, Cimprich B, Cleeland C, et al. NCCN Practice Guidelines for Cancer-Related Fatigue. Oncology (Williston Park). 2000; 14:151–161.2. Bruera E, Yennurajalingam S. Challenge of managing cancer-related fatigue. J Clin Oncol. 2010; 28:3671–3672.3. Ahlberg K, Ekman T, Gaston-Johansson F, Mock V. Assessment and management of cancer-related fatigue in adults. Lancet. 2003; 362:640–650.4. Curt GA. Impact of fatigue on quality of life in oncology patients. Semin Hematol. 2000; 37:4 Suppl 6. 14–17.5. Maughan TS, James RD, Kerr DJ, Ledermann JA, McArdle C, Seymour MT, et al. Comparison of survival, palliation, and quality of life with three chemotherapy regimens in metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2002; 359:1555–1563.6. Weis J. Cancer-related fatigue: prevalence, assessment and treatment strategies. Expert Rev Pharmacoecon Outcomes Res. 2011; 11:441–446.7. Cella D, Davis K, Breitbart W, Curt G. Fatigue Coalition. Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Oncol. 2001; 19:3385–3391.8. Ruffer JU, Flechtner H, Tralls P, Josting A, Sieber M, Lathan B, et al. Fatigue in long-term survivors of Hodgkin's lymphoma; a report from the German Hodgkin Lymphoma Study Group (GHSG). Eur J Cancer. 2003; 39:2179–2186.9. Vogelzang NJ, Breitbart W, Cella D, Curt GA, Groopman JE, Horning SJ, et al. Patient, caregiver, and oncologist perceptions of cancer-related fatigue: results of a tripart assessment survey. The Fatigue Coalition. Semin Hematol. 1997; 34:3 Suppl 2. 4–12.10. Singer S, Kuhnt S, Zwerenz R, Eckert K, Hofmeister D, Dietz A, et al. Age- and sex-standardised prevalence rates of fatigue in a large hospital-based sample of cancer patients. Br J Cancer. 2011; 105:445–451.11. Seyidova-Khoshknabi D, Davis MP, Walsh D. Review article: a systematic review of cancer-related fatigue measurement questionnaires. Am J Hosp Palliat Care. 2011; 28:119–129.12. Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995; 39:315–325.13. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993; 85:365–376.14. Schwarz R, Krauss O, Hinz A. Fatigue in the general population. Onkologie. 2003; 26:140–144.15. Schwarz R, Hinz A. Reference data for the quality of life questionnaire EORTC QLQ-C30 in the general German population. Eur J Cancer. 2001; 37:1345–1351.16. Hagelin CL, Wengstrom Y, Runesdotter S, Furst CJ. The psychometric properties of the Swedish Multidimensional Fatigue Inventory MFI-20 in four different populations. Acta Oncol. 2007; 46:97–104.17. Smets EM, Garssen B, Cull A, de Haes JC. Application of the multidimensional fatigue inventory (MFI-20) in cancer patients receiving radiotherapy. Br J Cancer. 1996; 73:241–245.18. Hinz A, Fleischer M, Brahler E, Wirtz H, Bosse-Henck A. Fatigue in patients with sarcoidosis, compared with the general population. Gen Hosp Psychiatry. 2011; 33:462–468.19. Kuhnt S, Ernst J, Singer S, Ruffer JU, Kortmann RD, Stolzenburg JU, et al. Fatigue in cancer survivors-prevalence and correlates. Onkologie. 2009; 32:312–317.20. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983; 67:361–370.21. Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Pers Soc Psychol. 1994; 67:1063–1078.22. Ware JE, Kosinski M, Dewey JE, Gandek B. How to score and interpret single-item health status measures: a manual for users of the SF-8™ Health Survey. Lincoln, RI: QualityMetric Incorporated;2001.23. Henrich G, Herschbach P. Questions on life satisfaction (FLZM): a short questionnaire for assessing subjective quality of life. Eur J Psychol Assess. 2000; 16:150–159.24. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Erlbaum;1988.25. Gerber LH, Stout N, McGarvey C, Soballe P, Shieh CY, Diao G, et al. Factors predicting clinically significant fatigue in women following treatment for primary breast cancer. Support Care Cancer. 2011; 19:1581–1591.26. Weis J, Arraras JI, Conroy T, Efficace F, Fleissner C, Gorog A, et al. Development of an EORTC quality of life phase III module measuring cancer-related fatigue (EORTC QLQ-FA13). Psychooncology. 2013; 22:1002–1007.27. Osoba D. What has been learned from measuring health-related quality of life in clinical oncology. Eur J Cancer. 1999; 35:1565–1570.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Impacts of Fatigue, Pain, Anxiety, and Depression on the Quality of Life in Patients with Breast Cancer
- Factors Affecting Sleep Quality in Women with Cancer Undergoing Radiotherapy
- Construct Validity of the Revised Piper Fatigue Scale in Korean Women With Breast Cancer
- Cancer-related Fatigue of Breast Cancer Survivors: Qualitative Research
- Influence of Spiritual Health and Fatigue on Depression in Breast Cancer Patients