J Gynecol Oncol.
2013 Jul;24(3):236-241. 10.3802/jgo.2013.24.3.236.
Disease progression and recurrence in women treated for vulvovaginal intraepithelial neoplasia
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Cantonal Hospital, Frauenfeld, Switzerland. mathias.fehr@stgag.ch
- 2Division of Gynecology, Department of Obstetrics and Gynecology, University Hospital, Berne, Switzerland.
- 3Division of Gynecology, Department of Obstetrics and Gynecology, University Hospital, Zurich, Switzerland.
- 4Department of Obstetrics and Gynecology, Cantonal Hospital Bruderholz, Basel, Switzerland.
- KMID: 2288530
- DOI: http://doi.org/10.3802/jgo.2013.24.3.236
Abstract
OBJECTIVE
The malignant potential of intraepithelial neoplasia of the vulva and vagina after treatment is not well defined. Our objective was to examine risk factors for recurrence and invasive disease.
METHODS
Four hundred sixty-four women with biopsy proven high-grade intraepithelial neoplasia of the vulva and vagina were identified in the electronic databases of four colposcopy clinics. Inclusion criteria were a follow-up of more than one year, no history of invasive cancer and no invasive cancer within the first year after initial treatment. We investigated the potential factors associated with recurrence and progression using a logistic regression analysis to estimate odds ratios (ORs) and 95% confidence intervals (CIs).
RESULTS
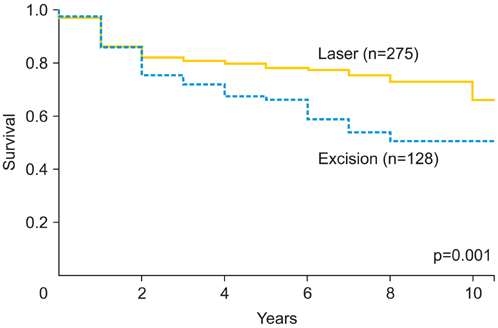
Of the 411 eligible patients, 123 patients (29.9%) recurred later than one year after initial treatment and 24 patients (5.8%) progressed to invasive disease. According to multivariate analyses, the risk factors associated with recurrence were multifocality (OR, 3.33; 95% CI, 2.02 to 5.51), immunosuppression (OR, 2.51; 95% CI, 1.09 to 5.81), excision as initial treatment (vs. laser evaporation; OR, 1.79; 95% CI, 1.11 to 2.91) and smoking (OR, 1.61; 95% CI, 1.02 to 2.55). Risk factors for progression to invasive disease were immunosuppression (OR, 4.00; 95% CI, 1.30 to 12.25), multifocality (OR, 3.05; 95% CI, 1.25 to 7.43) and smoking (OR, 2.97; 95% CI, 1.16 to 7.60), but not treatment modality.
CONCLUSION
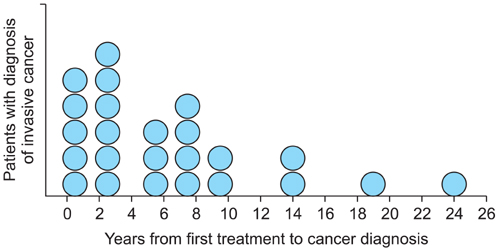
Laser evaporation combined with extensive biopsy is at least as efficacious as initial treatment of intraepithelial neoplasia with excision. Smoking is a risk factor for both recurrence and progression to invasive disease. Hence, smoking cessation should be advised and maintaining a long follow-up period due to late relapses is necessary.
Keyword
MeSH Terms
Figure
Reference
-
1. Judson PL, Habermann EB, Baxter NN, Durham SB, Virnig BA. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstet Gynecol. 2006; 107:1018–1022.2. Jacyntho CM, Giraldo PC, Horta AA, Grandelle R, Goncalves AK, Fonseca T, et al. Association between genital intraepithelial lesions and anal squamous intraepithelial lesions in HIV-negative women. Am J Obstet Gynecol. 2011; 205:115.e1–115.e5.3. Sideri M, Jones RW, Wilkinson EJ, Preti M, Heller DS, Scurry J, et al. Squamous vulvar intraepithelial neoplasia: 2004 modified terminology, ISSVD Vulvar Oncology Subcommittee. J Reprod Med. 2005; 50:807–810.4. Frega A, Sopracordevole F, Scirpa P, Biamonti A, Lorenzon L, Scarani S, et al. The re-infection rate of high-risk HPV and the recurrence rate of vulvar intraepithelial neoplasia (VIN) usual type after surgical treatment. Med Sci Monit. 2011; 17:CR532–CR535.5. van Seters M, van Beurden M, ten Kate FJ, Beckmann I, Ewing PC, Eijkemans MJ, et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod. N Engl J Med. 2008; 358:1465–1473.6. van Seters M, van Beurden M, de Craen AJ. Is the assumed natural history of vulvar intraepithelial neoplasia III based on enough evidence? A systematic review of 3322 published patients. Gynecol Oncol. 2005; 97:645–651.7. Committee on Gynecologic Practice of American College Obstetricians and Gynecologists. ACOG Committee opinion no. 509: management of vulvar intraepithelial neoplasia. Obstet Gynecol. 2011; 118:1192–1194.8. Jones RW, Rowan DM, Stewart AW. Vulvar intraepithelial neoplasia: aspects of the natural history and outcome in 405 women. Obstet Gynecol. 2005; 106:1319–1326.9. Stephenson RD, Denehy TR. Rapid spontaneous regression of acute-onset vulvar intraepithelial neoplasia 3 in young women: a case series. J Low Genit Tract Dis. 2012; 16:56–58.10. McFadden KM, Sharp L, Cruickshank ME. The prospective management of women with newly diagnosed vulval intraepithelial neoplasia: clinical outcome and quality of life. J Obstet Gynaecol. 2009; 29:749–753.11. Modesitt SC, Waters AB, Walton L, Fowler WC Jr, Van Le L. Vulvar intraepithelial neoplasia III: occult cancer and the impact of margin status on recurrence. Obstet Gynecol. 1998; 92:962–966.12. Herod JJ, Shafi MI, Rollason TP, Jordan JA, Luesley DM. Vulvar intraepithelial neoplasia: long term follow up of treated and untreated women. Br J Obstet Gynaecol. 1996; 103:446–452.13. Hillemanns P, Wang X, Staehle S, Michels W, Dannecker C. Evaluation of different treatment modalities for vulvar intraepithelial neoplasia (VIN): CO(2) laser vaporization, photodynamic therapy, excision and vulvectomy. Gynecol Oncol. 2006; 100:271–275.14. Kuppers V, Stiller M, Somville T, Bender HG. Risk factors for recurrent VIN: role of multifocality and grade of disease. J Reprod Med. 1997; 42:140–144.15. Sillman FH, Fruchter RG, Chen YS, Camilien L, Sedlis A, McTigue E. Vaginal intraepithelial neoplasia: risk factors for persistence, recurrence, and invasion and its management. Am J Obstet Gynecol. 1997; 176:93–99.16. van Beurden M, ten Kate FJ, Smits HL, Berkhout RJ, de Craen AJ, van der Vange N, et al. Multifocal vulvar intraepithelial neoplasia grade III and multicentric lower genital tract neoplasia is associated with transcriptionally active human papillomavirus. Cancer. 1995; 75:2879–2884.17. De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J Cancer. 2009; 124:1626–1636.18. Petry KU, Köchel H, Bode U, Schedel I, Niesert S, Glaubitz M, et al. Human papillomavirus is associated with the frequent detection of warty and basaloid high-grade neoplasia of the vulva and cervical neoplasia among immunocompromised women. Gynecol Oncol. 1996; 60:30–34.19. Dantal J, Hourmant M, Cantarovich D, Giral M, Blancho G, Dreno B, et al. Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. Lancet. 1998; 351:623–628.20. Dedes KJ, Beneder C, Samartzis N, Muller MD, Fink D, Fehr MK. Outcome of treated anogenital intraepithelial neoplasia among human immunodeficiency virus-infected women. J Reprod Med. 2008; 53:947–951.21. Massad LS, Xie X, Darragh T, Minkoff H, Levine AM, Watts DH, et al. Genital warts and vulvar intraepithelial neoplasia: natural history and effects of treatment and human immunodeficiency virus infection. Obstet Gynecol. 2011; 118:831–839.22. Castellsague X, Munoz N. Chapter 3: Cofactors in human papillomavirus carcinogenesis: role of parity, oral contraceptives, and tobacco smoking. J Natl Cancer Inst Monogr. 2003; 31:20–28.23. Sasson IM, Haley NJ, Hoffmann D, Wynder EL, Hellberg D, Nilsson S. Cigarette smoking and neoplasia of the uterine cervix: smoke constituents in cervical mucus. N Engl J Med. 1985; 312:315–316.24. Poppe WA, Ide PS, Drijkoningen MP, Lauweryns JM, Van Assche FA. Tobacco smoking impairs the local immunosurveillance in the uterine cervix. An immunohistochemical study. Gynecol Obstet Invest. 1995; 39:34–38.25. Barton SE, Maddox PH, Jenkins D, Edwards R, Cuzick J, Singer A. Effect of cigarette smoking on cervical epithelial immunity: a mechanism for neoplastic change? Lancet. 1988; 2:652–654.26. Hussain SK, Madeleine MM, Johnson LG, Du Q, Malkki M, Wilkerson HW, et al. Cervical and vulvar cancer risk in relation to the joint effects of cigarette smoking and genetic variation in interleukin 2. Cancer Epidemiol Biomarkers Prev. 2008; 17:1790–1799.27. Szarewski A, Jarvis MJ, Sasieni P, Anderson M, Edwards R, Steele SJ, et al. Effect of smoking cessation on cervical lesion size. Lancet. 1996; 347:941–943.28. Iversen T, Tretli S. Intraepithelial and invasive squamous cell neoplasia of the vulva: trends in incidence, recurrence, and survival rate in Norway. Obstet Gynecol. 1998; 91:969–972.29. Santoso JT, Crigger M, English E, Wan J, Likes W. Smoking cessation counseling in women with genital intraepithelial neoplasia. Gynecol Oncol. 2012; 125:716–719.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of the safety between cervical conization and hysterectomy for patients with cervical adenocarcinoma in situ
- The Effect of Topical Mitomycin C after Excisional Biopsy in Conjunctival-corneal Intraepithelial Neoplasia: Two cases
- Total vaginectomy for refractory vaginal intraepithelial neoplasia III of the vaginal vault
- Health Care Utilization in Women with Cervical Cancer and Cervical Intraepithelial Neoplasia
- High-Grade Prostatic Intraepithelial Neoplasia