J Clin Neurol.
2013 Jan;9(1):43-50. 10.3988/jcn.2013.9.1.43.
Reduced Frontal P3a Amplitude in Migraine Patients during the Pain-Free Period
- Affiliations
-
- 1Department of Neurology, Korea University Medical Center, Korea University College of Medicine, Seoul, Korea. jungky@korea.ac.kr
- 2Department of Neurology, Jeju Medical Center of Jeju Special Self-Governing Province, Jeju, Korea.
- 3BK21 Program for Biomedical Science, Korea University College of Medicine, Seoul, Korea.
- 4Department of Psychology, Sungshin Women's University, Seoul, Korea.
- 5Department of Biomedical Engineering, College of Health Science, Yonsei University, Wonju, Korea.
- 6Department of Biomedical Engineering, Hanyang University, Seoul, Korea.
- KMID: 2287570
- DOI: http://doi.org/10.3988/jcn.2013.9.1.43
Abstract
- BACKGROUND AND PURPOSE
Neuropsychological and neuroimaging studies both suggest that frontal lobe dysfunction is present in migraineurs. Since P3a abnormalities manifest in other diseases associated with attention problems, such as attention deficit hyperactivity disorder, we hypothesized that migraine patients have P3a abnormalities, particularly in the frontal region.
METHODS
Event-related potentials were measured using a passive auditory oddball paradigm in 16 female migraineurs (aged 22.9+/-2.0 years, mean+/-SD) during the interictal period and in 16 age-matched healthy females (22.6+/-2.0 years). The amplitudes and latencies were analyzed independently using repeated-measures analysis of variance. Nonparametric statistical testing using a cluster-level randomization method was performed to localize the abnormalities.
RESULTS
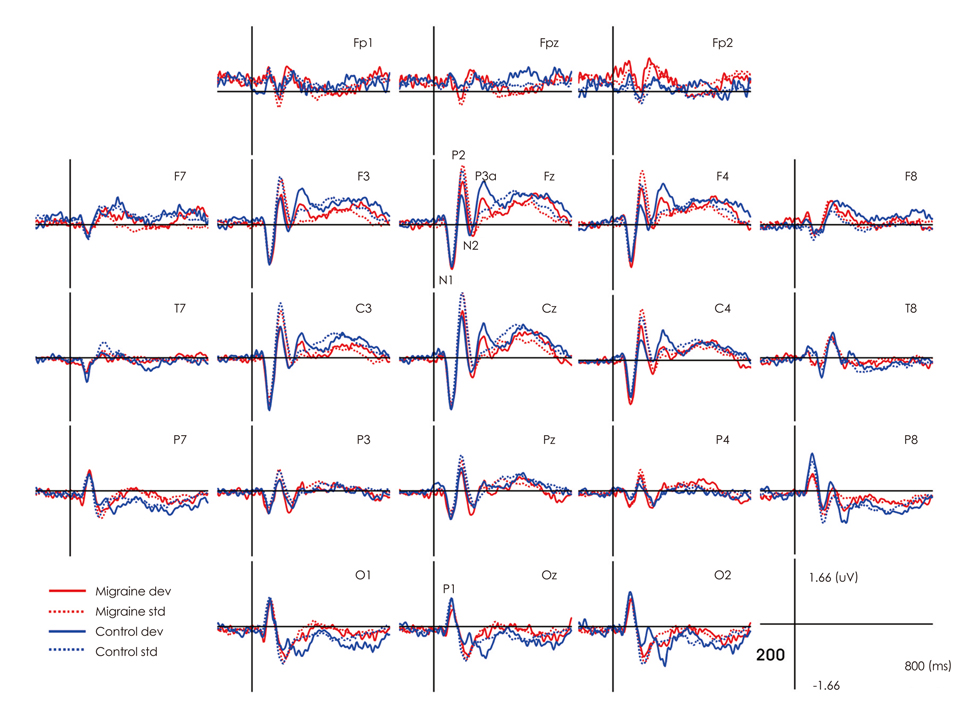
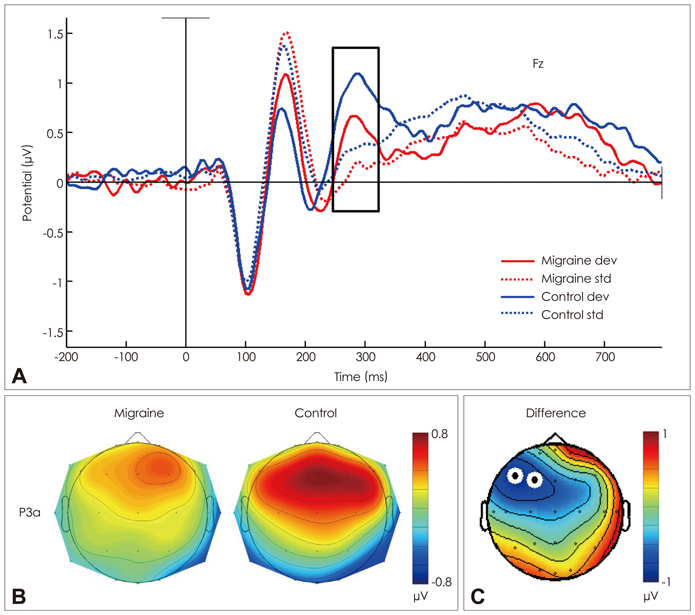
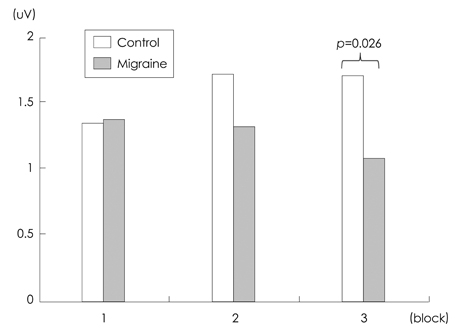
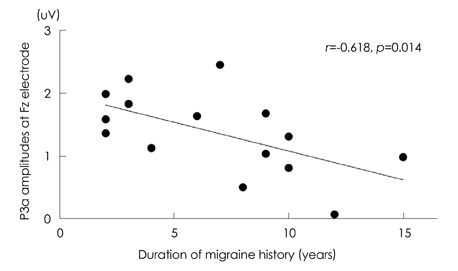
The mean P3a amplitude at frontal areas during the third trials was significantly lower in migraineurs (1.06 microV) than in controls (1.69 microV, p=0.026). P3a amplitudes were negatively correlated with the duration of the migraine history (r=-0.618, p=0.014). Cluster-based nonparametric statistical analysis showed that the amplitudes over left frontal areas were significantly lower in migraine patients than in controls.
CONCLUSIONS
A reduced P3a amplitude of migraineurs reflects attentional deficits and frontal dysfunction. The negative correlation between P3a amplitude and the duration of the migraine history suggests that attentional deficits and frontal dysfunction are either the cause or the result of headache.
Keyword
MeSH Terms
Figure
Reference
-
1. Lipton RB, Bigal ME. Migraine: epidemiology, impact, and risk factors for progression. Headache. 2005. 45:Suppl 1. S3–S13.
Article2. Schneider F, Karoly P. Conceptions of the pain experience: the emergence of multidimensional models and their implications for contemporary clinical-practice. Clin Psychol Rev. 1983. 3:61–86.
Article3. Hasenbring M. Attentional control of pain and the process of chronification. Prog Brain Res. 2000. 129:525–534.
Article4. Goadsby PJ. Is migraine a progressive disorder? Considering the clinical implications of new research data on migraine and brain lesions. Med J Aust. 2005. 182:103–104.5. Lipton RB, Bigal ME. Looking to the future: research designs for study of headache disease progression. Headache. 2008. 48:58–66.
Article6. Aguggia M, Saracco MG. Pathophysiology of migraine chronification. Neurol Sci. 2010. 31:Suppl 1. S15–S17.
Article7. Le Pira F, Zappalà G, Giuffrida S, Lo Bartolo ML, Reggio E, Morana R, et al. Memory disturbances in migraine with and without aura: a strategy problem? Cephalalgia. 2000. 20:475–478.
Article8. Calandre EP, Bembibre J, Arnedo ML, Becerra D. Cognitive disturbances and regional cerebral blood flow abnormalities in migraine patients: their relationship with the clinical manifestations of the illness. Cephalalgia. 2002. 22:291–302.
Article9. Waldie KE, Hausmann M, Milne BJ, Poulton R. Migraine and cognitive function: a life-course study. Neurology. 2002. 59:904–908.
Article10. Gómez-Beldarrain M, Carrasco M, Bilbao A, García-Moncó JC. Orbitofrontal dysfunction predicts poor prognosis in chronic migraine with medication overuse. J Headache Pain. 2011. 12:459–466.
Article11. Camarda C, Monastero R, Pipia C, Recca D, Camarda R. Interictal executive dysfunction in migraineurs without aura: relationship with duration and intensity of attacks. Cephalalgia. 2007. 27:1094–1100.
Article12. Schmitz N, Arkink EB, Mulder M, Rubia K, Admiraal-Behloul F, Schoonman GG, et al. Frontal lobe structure and executive function in migraine patients. Neurosci Lett. 2008. 440:92–96.
Article13. Kim JH, Suh SI, Seol HY, Oh K, Seo WK, Yu SW, et al. Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia. 2008. 28:598–604.
Article14. Afridi SK, Giffin NJ, Kaube H, Friston KJ, Ward NS, Frackowiak RS, et al. A positron emission tomographic study in spontaneous migraine. Arch Neurol. 2005. 62:1270–1275.
Article15. Kim JH, Kim S, Suh SI, Koh SB, Park KW, Oh K. Interictal metabolic changes in episodic migraine: a voxel-based FDG-PET study. Cephalalgia. 2010. 30:53–61.
Article16. Schmitz N, Admiraal-Behloul F, Arkink EB, Kruit MC, Schoonman GG, Ferrari MD, et al. Attack frequency and disease duration as indicators for brain damage in migraine. Headache. 2008. 48:1044–1055.
Article17. Woodman GF. A brief introduction to the use of event-related potentials in studies of perception and attention. Atten Percept Psychophys. 2010. 72:2031–2046.
Article18. Polich J, Kok A. Cognitive and biological determinants of P300: an integrative review. Biol Psychol. 1995. 41:103–146.
Article19. Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007. 118:2128–2148.
Article20. Volpe U, Mucci A, Bucci P, Merlotti E, Galderisi S, Maj M. The cortical generators of P3a and P3b: a LORETA study. Brain Res Bull. 2007. 73:220–230.
Article21. Baving L, Rellum T, Laucht M, Schmidt MH. Children with oppositional-defiant disorder display deviant attentional processing independent of ADHD symptoms. J Neural Transm. 2006. 113:685–693.
Article22. Banaschewski T, Brandeis D, Heinrich H, Albrecht B, Brunner E, Rothenberger A. Association of ADHD and conduct disorder--brain electrical evidence for the existence of a distinct subtype. J Child Psychol Psychiatry. 2003. 44:356–376.
Article23. Anderson NE, Baldridge RM, Stanford MS. P3a amplitude predicts successful treatment program completion in substance-dependent individuals. Subst Use Misuse. 2011. 46:669–677.
Article24. Wang W, Schoenen J. Interictal potentiation of passive "oddball" auditory event-related potentials in migraine. Cephalalgia. 1998. 18:261–265.
Article25. Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004. 24:Suppl 1. 9–160.26. Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004. 134:9–21.
Article27. Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011. 2011:156869.
Article28. Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007. 164:177–190.
Article29. Vannatta K, Getzoff EA, Powers SW, Noll RB, Gerhardt CA, Hershey AD. Multiple perspectives on the psychological functioning of children with and without migraine. Headache. 2008. 48:994–1004.
Article30. Moutran AR, Villa TR, Diaz LA, Noffs MH, Pinto MM, Gabbai AA, et al. Migraine and cognition in children: a controlled study. Arq Neuropsiquiatr. 2011. 69:192–195.
Article31. Mulder EJ, Linssen WH, Passchier J, Orlebeke JF, de Geus EJ. Interictal and postictal cognitive changes in migraine. Cephalalgia. 1999. 19:557–565. discussion 541.
Article32. Clemens B, Bánk J, Piros P, Bessenyei M, Veto S, Tóth M, et al. Three-dimensional localization of abnormal EEG activity in migraine: a low resolution electromagnetic tomography (LORETA) study of migraine patients in the pain-free interval. Brain Topogr. 2008. 21:36–42.
Article33. Petrovic P, Petersson KM, Ghatan PH, Stone-Elander S, Ingvar M. Pain-related cerebral activation is altered by a distracting cognitive task. Pain. 2000. 85:19–30.
Article34. Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, Conrad B, et al. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain--an fMRI analysis. Pain. 2004. 109:399–408.
Article35. Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003. 126:1079–1091.
Article36. Brighina F, Piazza A, Vitello G, Aloisio A, Palermo A, Daniele O, et al. rTMS of the prefrontal cortex in the treatment of chronic migraine: a pilot study. J Neurol Sci. 2004. 227:67–71.
Article37. Zakzanis KK, Mraz R, Graham SJ. An fMRI study of the Trail Making Test. Neuropsychologia. 2005. 43:1878–1886.
Article38. Faber PL, Tei S, Chen C, Hsiao P, Lehmann D. 3. Brain LORETA functional imaging, EEG spectral power, and self-rated headache pain. Clin Neurophysiol. 2011. 122:e2.
Article39. Parisi P, Verrotti A, Paolino MC, Urbano A, Bernabucci M, Castaldo R, et al. Headache and cognitive profile in children: a cross-sectional controlled study. J Headache Pain. 2010. 11:45–51.
Article40. Eccleston C, Crombez G. Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychol Bull. 1999. 125:356–366.
Article41. Houlihan ME, McGrath PJ, Connolly JF, Stroink G, Allen Finley G, Dick B, et al. Assessing the effect of pain on demands for attentional resources using ERPs. Int J Psychophysiol. 2004. 51:181–187.
Article42. Seminowicz DA, Davis KD. Pain enhances functional connectivity of a brain network evoked by performance of a cognitive task. J Neurophysiol. 2007. 97:3651–3659.
Article43. Seidel S, Hartl T, Weber M, Matterey S, Paul A, Riederer F, et al. Quality of sleep, fatigue and daytime sleepiness in migraine - a controlled study. Cephalalgia. 2009. 29:662–669.
Article44. Kruit MC, Launer LJ, Overbosch J, van Buchem MA, Ferrari MD. Iron accumulation in deep brain nuclei in migraine: a population-based magnetic resonance imaging study. Cephalalgia. 2009. 29:351–359.
Article45. Valeriani M, Galli F, Tarantino S, Graceffa D, Pignata E, Miliucci R, et al. Correlation between abnormal brain excitability and emotional symptomatology in paediatric migraine. Cephalalgia. 2009. 29:204–213.
Article46. Siniatchkin M, Kropp P, Gerber WD. What kind of habituation is impaired in migraine patients? Cephalalgia. 2003. 23:511–518.
Article47. Evers S, Quibeldey F, Grotemeyer KH, Suhr B, Husstedt IW. Dynamic changes of cognitive habituation and serotonin metabolism during the migraine interval. Cephalalgia. 1999. 19:485–491.
Article48. Evers S, Bauer B, Grotemeyer KH, Kurlemann G, Husstedt IW. Event-related potentials (P300) in primary headache in childhood and adolescence. J Child Neurol. 1998. 13:322–326.
Article49. Evers S, Bauer B, Suhr B, Husstedt IW, Grotemeyer KH. Cognitive processing in primary headache: a study on event-related potentials. Neurology. 1997. 48:108–113.
Article50. Demarquay G, Caclin A, Brudon F, Fischer C, Morlet D. Exacerbated attention orienting to auditory stimulation in migraine patients. Clin Neurophysiol. 2011. 122:1755–1763.
Article51. Zohsel K, Hohmeister J, Flor H, Hermann C. Altered pain processing in children with migraine: an evoked potential study. Eur J Pain. 2008. 12:1090–1101.
Article52. Chen W, Shen X, Liu X, Luo B, Liu Y, Yu R, et al. Passive paradigm single-tone elicited ERPs in tension-type headaches and migraine. Cephalalgia. 2007. 27:139–144.
Article53. Gerber WD, Stephani U, Kirsch E, Kropp P, Siniatchkin M. Slow cortical potentials in migraine families are associated with psychosocial factors. J Psychosom Res. 2002. 52:215–222.
Article54. Buodo G, Palomba D, Sarlo M, Naccarella C, Battistella PA. Auditory event-related potentials and reaction times in migraine children. Cephalalgia. 2004. 24:554–563.
Article55. Siniatchkin M, Kirsch E, Kropp P, Stephani U, Gerber WD. Slow cortical potentials in migraine families. Cephalalgia. 2000. 20:881–892.
Article56. Polich J, McIsaac HK. Comparison of auditory P300 habituation from active and passive conditions. Int J Psychophysiol. 1994. 17:25–34.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Reduced Frontal P3a Amplitude in Migraine Patients during the Pain-Free Period
- Event-Related Potential P3a and P3b using 3-Stimulus Auditory "Oddball" Paradigm in the Patients with Schizophrenia
- Clinical Course and Prognosis of Migraine Headache in Childhood and Adolescence
- Are there network differences between the ipsilateral and contralateral hemispheres of pain in patients with episodic migraine without aura?
- Efficacy of a Respiratory Training System on the Regularity of Breathing