J Clin Neurol.
2014 Oct;10(4):289-295. 10.3988/jcn.2014.10.4.289.
Cardiotoxicity of Mitoxantrone Treatment in a German Cohort of 639 Multiple Sclerosis Patients
- Affiliations
-
- 1Department of Neurology, Focus Program Translational Neuroscience, Rhine Main Neuroscience Network, University Medical Center, Johannes Gutenberg University of Mainz, Mainz, Germany. luessi@uni-mainz.de
- 2Department of Neurology, St. Josef-Hospital, Ruhr University of Bochum, Bochum, Germany.
- 3Institute of Medical Biostatistics, Epidemiology and Informatics, University Medical Center, Johannes Gutenberg University of Mainz, Mainz, Germany.
- KMID: 2287507
- DOI: http://doi.org/10.3988/jcn.2014.10.4.289
Abstract
- BACKGROUND AND PURPOSE
The aim of this study was to elucidate the role of therapy-related cardiotoxicity in multiple sclerosis (MS) patients treated with mitoxantrone and to identify potential predictors for individual risk assessment.
METHODS
Within a multicenter retrospective cohort design, cardiac side effects attributed to mitoxantrone were analyzed in 639 MS patients at 2 MS centers in Germany. Demographic, disease, treatment, and follow-up data were collected from hospital records. Patients regularly received cardiac monitoring during the treatment phase.
RESULTS
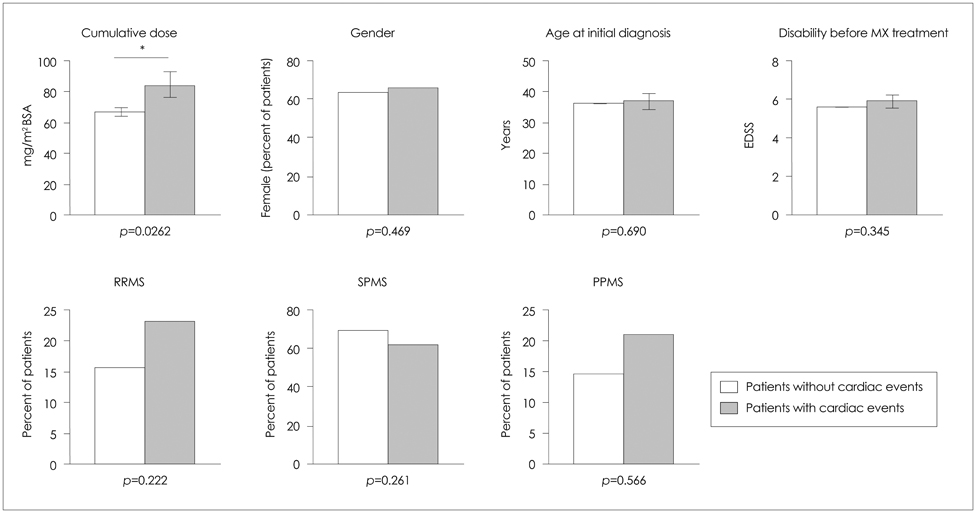
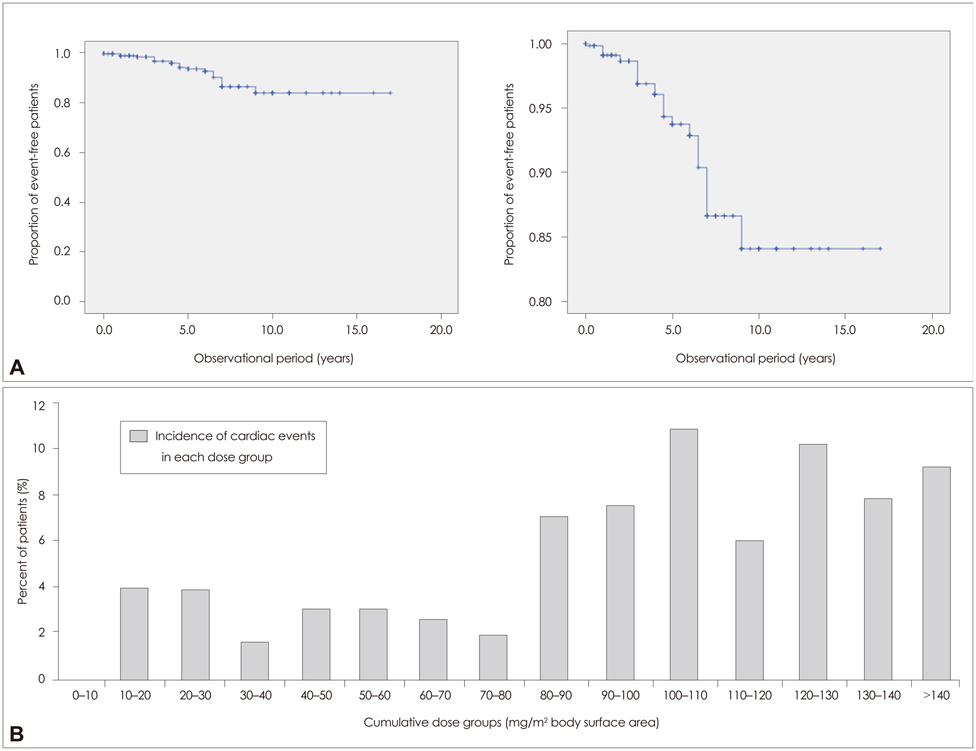
None of the patients developed symptomatic congestive heart failure. However, the frequency of patients experiencing cardiac dysfunction of milder forms after mitoxantrone therapy was 4.1% (26 patients) among all patients. Analyses of the risk for cardiotoxicity revealed that cumulative dose exposure was the only statistically relevant risk factor associated with cardiac dysfunction.
CONCLUSIONS
The number of patients developing subclinical cardiac dysfunction below the maximum recommended cumulative dose is higher than was initially assumed. Interestingly, a subgroup of patients was identified who experienced cardiac dysfunction shortly after initiation of mitoxantrone and who received a low cumulative dose. Therefore, each administration of mitoxantrone should include monitoring of cardiac function to enhance the treatment safety for patients and to allow for early detection of any side effects, especially in potential high-risk subgroups (as determined genetically).
MeSH Terms
Figure
Reference
-
1. Neuhaus O, Kieseier BC, Hartung HP. Mechanisms of mitoxantrone in multiple sclerosis--what is known? J Neurol Sci. 2004; 223:25–27.
Article2. Hartung HP, Gonsette R, König N, Kwiecinski H, Guseo A, Morrissey SP, et al. Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet. 2002; 360:2018–2025.
Article3. Arlin ZA, Silver R, Cassileth P, Armentrout S, Gams R, Daghestani A, et al. Phase I-II trial of mitoxantrone in acute leukemia. Cancer Treat Rep. 1985; 69:61–64.4. Mather FJ, Simon RM, Clark GM, Von Hoff DD. Cardiotoxicity in patients treated with mitoxantrone: Southwest Oncology Group phase II studies. Cancer Treat Rep. 1987; 71:609–613.5. Praga C, Beretta G, Vigo PL, Lenaz GR, Pollini C, Bonadonna G, et al. Adriamycin cardiotoxicity: a survey of 1273 patients. Cancer Treat Rep. 1979; 63:827–834.6. Ghalie RG, Mauch E, Edan G, Hartung HP, Gonsette RE, Eisenmann S, et al. A study of therapy-related acute leukaemia after mitoxantrone therapy for multiple sclerosis. Mult Scler. 2002; 8:441–445.
Article7. Rivera VM, Jeffery DR, Weinstock-Guttman B, Bock D, Dangond F. Results from the 5-year, phase IV RENEW (Registry to Evaluate Novantrone Effects in Worsening Multiple Sclerosis) study. BMC Neurol. 2013; 13:80.
Article8. Le Page E, Leray E, Edan G. French Mitoxantrone Safety Group. Long-term safety profile of mitoxantrone in a French cohort of 802 multiple sclerosis patients: a 5-year prospective study. Mult Scler. 2011; 17:867–875.
Article9. Goffette S, van Pesch V, Vanoverschelde JL, Morandini E, Sindic CJ. Severe delayed heart failure in three multiple sclerosis patients previously treated with mitoxantrone. J Neurol. 2005; 252:1217–1222.
Article10. Zingler VC, Nabauer M, Jahn K, Gross A, Hohlfeld R, Brandt T, et al. Assessment of potential cardiotoxic side effects of mitoxantrone in patients with multiple sclerosis. Eur Neurol. 2005; 54:28–33.
Article11. Kingwell E, Koch M, Leung B, Isserow S, Geddes J, Rieckmann P, et al. Cardiotoxicity and other adverse events associated with mitoxantrone treatment for MS. Neurology. 2010; 74:1822–1826.
Article12. Paul F, Dörr J, Würfel J, Vogel HP, Zipp F. Early mitoxantrone-induced cardiotoxicity in secondary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry. 2007; 78:198–200.
Article13. Edan G, Miller D, Clanet M, Confavreux C, Lyon-Caen O, Lubetzki C, et al. Therapeutic effect of mitoxantrone combined with methylprednisolone in multiple sclerosis: a randomised multicentre study of active disease using MRI and clinical criteria. J Neurol Neurosurg Psychiatry. 1997; 62:112–118.
Article14. Dörr J, Bitsch A, Schmailzl KJ, Chan A, von Ahsen N, Hummel M, et al. Severe cardiac failure in a patient with multiple sclerosis following low-dose mitoxantrone treatment. Neurology. 2009; 73:991–993.
Article15. Xu X, Persson HL, Richardson DR. Molecular pharmacology of the interaction of anthracyclines with iron. Mol Pharmacol. 2005; 68:261–271.
Article16. Millefiorini E, Gasperini C, Pozzilli C, D'Andrea F, Bastianello S, Trojano M, et al. Randomized placebo-controlled trial of mitoxantrone in relapsing-remitting multiple sclerosis: 24-month clinical and MRI outcome. J Neurol. 1997; 244:153–159.
Article17. Rossato LG, Costa VM, de Pinho PG, Arbo MD, de Freitas V, Vilain L, et al. The metabolic profile of mitoxantrone and its relation with mitoxantrone-induced cardiotoxicity. Arch Toxicol. 2013; 87:1809–1820.
Article18. Cotte S, von Ahsen N, Kruse N, Huber B, Winkelmann A, Zettl UK, et al. ABC-transporter gene-polymorphisms are potential pharmacogenetic markers for mitoxantrone response in multiple sclerosis. Brain. 2009; 132(Pt 9):2517–2530.
Article19. Hasan SK, Buttari F, Ottone T, Voso MT, Hohaus S, Marasco E, et al. Risk of acute promyelocytic leukemia in multiple sclerosis: coding variants of DNA repair genes. Neurology. 2011; 76:1059–1065.
Article20. Chan A, Lo-Coco F. Mitoxantrone-related acute leukemia in MS: an open or closed book? Neurology. 2013; 80:1529–1533.
Article21. Bernitsas E, Wei W, Mikol DD. Suppression of mitoxantrone cardiotoxicity in multiple sclerosis patients by dexrazoxane. Ann Neurol. 2006; 59:206–209.
Article22. Weilbach FX, Chan A, Toyka KV, Gold R. The cardioprotector dexrazoxane augments therapeutic efficacy of mitoxantrone in experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2004; 135:49–55.
Article23. U.S. Food and Drug Administration [Internet]. Mitoxantrone Hydrochloride (marketed as Novantrone and generics) - Healthcare Professional Sheet text version. Rockville, MD: U.S. Food and Drug Administration;cited 2014 Jan 1. Available from: http://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm126445.htm.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multiple Sclerosis
- Risk of Trastuzumab-Related Cardiotoxicity in Early Breast Cancer Patients: A Prospective Observational Study
- Chemotherapy with mitoxantrone and etoposide in patients with highly refractory acute leukemia
- Mitoxantrone and cytosine arabinoside in adult patients with refractory and relapsed acute leukemia
- Pathophysiology and preventive strategies of anthracycline-induced cardiotoxicity