J Breast Cancer.
2012 Mar;15(1):65-70. 10.4048/jbc.2012.15.1.65.
Classification of Metastatic versus Non-Metastatic Axillary Nodes in Breast Cancer Patients: Value of Cortex-Hilum Area Ratio with Ultrasound
- Affiliations
-
- 1Department of Radiology, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea. seoboky@korea.ac.kr
- 2Department of Radiology, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Radiology, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea.
- 4Department of Radiology, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea.
- KMID: 2286475
- DOI: http://doi.org/10.4048/jbc.2012.15.1.65
Abstract
- PURPOSE
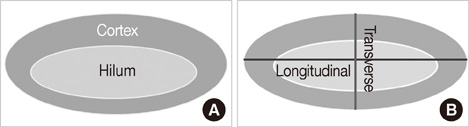
To investigate the significance of the cortex-hilum (CH) area ratio and longitudinal-transverse (LT) axis ratio and the blood flow pattern for diagnosis of metastatic axillary lymph nodes by ultrasound in breast cancer patients.
METHODS
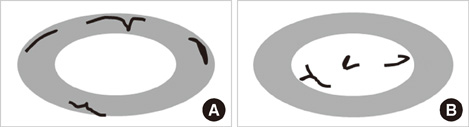
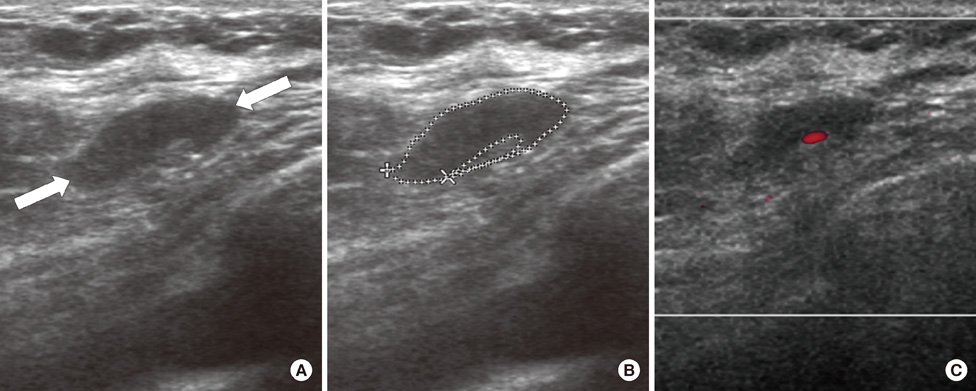
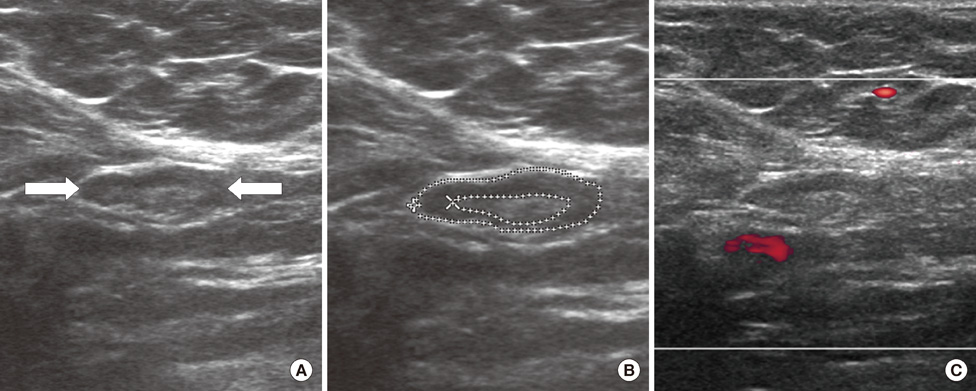
From October 2005 to July 2006, we prospectively evaluated axillary nodes with ultrasound in 205 consecutive patients who had category 4B, 4C or 5 breast lesions according to the Breast Imaging Reporting and Data System-Ultrasound (BI-RADS-Ultrasound(R)). Among the 205, there were 24 patients who had pathologic verification of breast cancer and axillary lymph node status. For a total of 80 axillary nodes we measured the areas of the cortex and hilum of lymph nodes and calculated the area ratio. We also measured the length of the longitudinal and transverse axis of the lymph nodes and calculated the length ratio. We evaluated the blood flow pattern on power Doppler imaging and classified each lymph node into a central or peripheral pattern. Diagnostic performance was analyzed according to positive criteria for lymph node metastasis (CH area ratio >2, LT axis ratio <2, peripheral type on power Doppler imaging).
RESULTS
The sensitivity of the CH area ratio was superior to that of the LT axis ratio (94.1% vs. 82.3%, p=0.031) and to that of the blood flow pattern (94.1% vs. 29.4%, p=0.009). For specificity, all three evaluating parameters had high values (89.1-95.6%) and no significant differences were found (p=0.121). The CH area ratio had a better positive predictive value than the LT axis ratio (94.1% vs. 80.0%, p=0.030) and power Doppler imaging (94.1% vs. 66.6%, p=0.028). For the negative predictive value, the CH area ratio was superior to the LT axis ratio (95.6% vs. 86.6%, p=0.035) and the blood flow pattern (95.6% vs. 63.0%, p=0.027).
CONCLUSION
We recommend the CH area ratio of an axillary lymph node on ultrasound as a quantitative indicator for the classification of lymph nodes. The CH area ratio can improve diagnostic performance when compared with the LT axis ratio or blood flow pattern.
Keyword
MeSH Terms
Figure
Reference
-
1. Banerjee M, George J, Song EY, Roy A, Hryniuk W. Tree-based model for breast cancer prognostication. J Clin Oncol. 2004. 22:2567–2575.
Article2. Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989. 63:181–187.
Article3. Reynolds C, Mick R, Donohue JH, Grant CS, Farley DR, Callans LS, et al. Sentinel lymph node biopsy with metastasis: can axillary dissection be avoided in some patients with breast cancer? J Clin Oncol. 1999. 17:1720–1726.
Article4. McMasters KM, Giuliano AE, Ross MI, Reintgen DS, Hunt KK, Byrd DR, et al. Sentinel-lymph-node biopsy for breast cancer: not yet the standard of care. N Engl J Med. 1998. 339:990–995.
Article5. de Kanter AY, van Eijck CH, van Geel AN, Kruijt RH, Henzen SC, Paul MA, et al. Multicentre study of ultrasonographically guided axillary node biopsy in patients with breast cancer. Br J Surg. 1999. 86:1459–1462.
Article6. Fraile M, Rull M, Julián FJ, Fusté F, Barnadas A, Llatjós M, et al. Sentinel node biopsy as a practical alternative to axillary lymph node dissection in breast cancer patients: an approach to its validity. Ann Oncol. 2000. 11:701–705.
Article7. Kumar R, Jana S, Heiba SI, Dakhel M, Axelrod D, Siegel B, et al. Retrospective analysis of sentinel node localization in multifocal, multicentric, palpable, or nonpalpable breast cancer. J Nucl Med. 2003. 44:7–10.8. Michel SC, Keller TM, Fröhlich JM, Fink D, Caduff R, Seifert B, et al. Preoperative breast cancer staging: MR imaging of the axilla with ultrasmall superparamagnetic iron oxide enhancement. Radiology. 2002. 225:527–536.
Article9. Tate JJ, Lewis V, Archer T, Guyer PG, Royle GT, Taylor I. Ultrasound detection of axillary lymph node metastases in breast cancer. Eur J Surg Oncol. 1989. 15:139–141.10. Damera A, Evans AJ, Cornford EJ, Wilson AR, Burrell HC, James JJ, et al. Diagnosis of axillary nodal metastases by ultrasound-guided core biopsy in primary operable breast cancer. Br J Cancer. 2003. 89:1310–1313.
Article11. Lernevall A. Imaging of axillary lymph nodes. Acta Oncol. 2000. 39:277–281.
Article12. Yang WT, Metreweli C, Lam PK, Chang J. Benign and malignant breast masses and axillary nodes: evaluation with echo-enhanced color power Doppler US. Radiology. 2001. 220:795–802.
Article13. Abe H, Schmidt RA, Sennett CA, Shimauchi A, Newstead GM. US-guided core needle biopsy of axillary lymph nodes in patients with breast cancer: why and how to do it. Radiographics. 2007. 27:Suppl 1. S91–S99.
Article14. American College of Radiology, BI-RADS Committee. ACR BI-RADS Breast Imaging and Reporting Data System: Breast Imaging Atlas. 2003. 4th ed. Reston: American College of Radiology.15. Sacre RA. Clinical evaluation of axillar lymph nodes compared to surgical and pathological findings. Eur J Surg Oncol. 1986. 12:169–173.16. Specht MC, Fey JV, Borgen PI, Cody HS 3rd. Is the clinically positive axilla in breast cancer really a contraindication to sentinel lymph node biopsy? J Am Coll Surg. 2005. 200:10–14.
Article17. Alvarez S, Añorbe E, Alcorta P, López F, Alonso I, Cortés J. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: a systematic review. AJR Am J Roentgenol. 2006. 186:1342–1348.
Article18. Feu J, Tresserra F, Fábregas R, Navarro B, Grases PJ, Suris JC, et al. Metastatic breast carcinoma in axillary lymph nodes: in vitro US detection. Radiology. 1997. 205:831–835.
Article19. Esen G, Gurses B, Yilmaz MH, Ilvan S, Ulus S, Celik V, et al. Gray scale and power Doppler US in the preoperative evaluation of axillary metastases in breast cancer patients with no palpable lymph nodes. Eur Radiol. 2005. 15:1215–1223.
Article20. Cho N, Moon WK, Han W, Park IA, Cho J, Noh DY. Preoperative sonographic classification of axillary lymph nodes in patients with breast cancer: node-to-node correlation with surgical histology and sentinel node biopsy results. AJR Am J Roentgenol. 2009. 193:1731–1737.
Article21. Chang MC, Crystal P, Colgan TJ. The evolving role of axillary lymph node fine-needle aspiration in the management of carcinoma of the breast. Cancer Cytopathol. 2011. 119:328–334.
Article22. Rao R, Lilley L, Andrews V, Radford L, Ulissey M. Axillary staging by percutaneous biopsy: sensitivity of fine-needle aspiration versus core needle biopsy. Ann Surg Oncol. 2009. 16:1170–1175.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Value of Ultrasonographic Detection for Metastatic Axillary Lymph Nodes in Breast Cancer
- Metastatic Axillary Lymph Nodes in Breast Cancer: Ultrasonographic Findings
- Efficacy of Ultrasound-Guided Core Needle Biopsy in Detecting Metastatic Axillary Lymph Nodes in Breast Cancer
- The Impact of the Ratio of Positive Nodes to Removed Nodes on Recurrence and Overall Survival in Node Positive Breast Cancer Patients
- Ipsilateral Lymphadenopathy After COVID-19 Vaccination in Patients With Newly Diagnosed Breast Cancer