J Breast Cancer.
2013 Jun;16(2):184-192. 10.4048/jbc.2013.16.2.184.
Prognostic Factors Related to Surgical Outcome of Liver Metastases of Breast Cancer
- Affiliations
-
- 1Department of Surgery, Naval Hospital of Varna, Varna, Bulgaria. danielkostov@abv.bg
- 2Clinic of Surgery, Specialized Hospital for Oncologic Diseases of Varna, Varna, Bulgaria.
- KMID: 2286399
- DOI: http://doi.org/10.4048/jbc.2013.16.2.184
Abstract
- PURPOSE
The role of hepatectomy for patients with liver metastases of breast cancer (LMBC) remains controversial. The purpose of this study is to share our experience with hepatic resection in a relatively unselected group of patients with LMBC and analyse the prognostic factors and indications for surgery.
METHODS
In 2000 to 2006, 42 female patients with a mean age of 58.2 years (range, 39 to 69 years) with LMBC diagnosed by means of abdominal ultrasound, computed tomography and/or magnetic resonance imaging in the hospital. They were considered for surgery because of limited comorbidities, presence of seven or fewer liver tumors and absence of (or limited and stable) extrahepatic disease on preoperative imaging. Patients' demographics, metastatic characteristics as well as clinical and operative parameters were being studied. Overall actuarial 1-, 3-, and 5-year survival rates were calculated since the hepatic resection onwards using the Kaplan-Meier method.
RESULTS
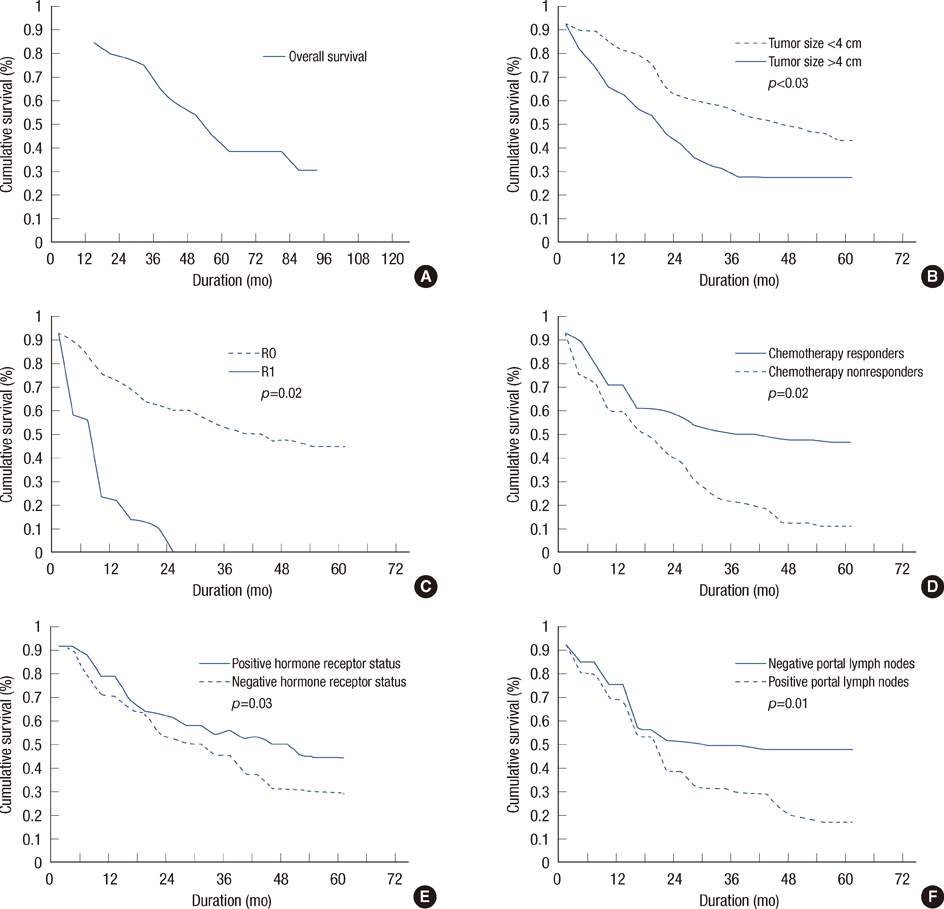
Metastatic tumor size of < or =4 cm (p=0.03), R0 resection (p=0.02), negative portal lymph nodes (p=0.01), response to chemotherapy (p=0.02), and positive hormone receptor status (p=0.03) were associated with better survival outcomes on univariate analysis. However, it did not show survival benefits on multivariate analysis. The disease-free survival and overall survival are 29.40 and 43 months, respectively. The 1-, 3- and 5-year survival rates were 84.61%, 64.11%, and 38.45%, respectively.
CONCLUSION
Selected patients with isolated LMBC may benefit from surgical management; although, indications remain unclear and the risks may outweigh the benefits in patients with a generally poor prognosis. Improvements in preoperative staging and progressive application of new multimodality treatments will be the key to improved survival rates in this severe disease. The careful selection of patients is associated with a satisfactory long-term survival rate.
Keyword
MeSH Terms
Figure
Reference
-
1. Li XP, Meng ZQ, Guo WJ, Li J. Treatment for liver metastases from breast cancer: results and prognostic factors. World J Gastroenterol. 2005; 11:3782–3787.
Article2. Elias D, Di Pietroantonio D. Surgery for liver metastases from breast cancer. HPB (Oxford). 2006; 8:97–99.
Article3. Elias D, Lasser P, Spielmann M, May-Levin F, el Malt O, Thomas H, et al. Surgical and chemotherapeutic treatment of hepatic metastases from carcinoma of the breast. Surg Gynecol Obstet. 1991; 172:461–464.4. Díaz R, Santaballa A, Munárriz B, Calderero V. Hepatic resection in breast cancer metastases: should it be considered standard treatment? Breast. 2004; 13:254–258.
Article5. Piccart MJ, Awada A, Hamilton A. Integration of new therapies into management of metastatic breast cancer: a focus on chemotherapy, treatment selection through use of molecular markers and newly developed biologic therapies in late clinical development. Am Soc Clin Oncol Educ Book. 1999; 526–539.6. Aapro MS. Combining new agents with anthracyclines in metastatic breast cancer: an overview of recent findings. Semin Oncol. 1999; 26:1 Suppl 3. 17–21.7. Cardoso F, Costa A, Norton L, Cameron D, Cufer T, Fallowfield L, et al. 1st International consensus guidelines for advanced breast cancer (ABC 1). Breast. 2012; 21:242–252.
Article8. Vlastos G, Smith DL, Singletary SE, Mirza NQ, Tuttle TM, Popat RJ, et al. Long-term survival after an aggressive surgical approach in patients with breast cancer hepatic metastases. Ann Surg Oncol. 2004; 11:869–874.
Article9. Malassagne B, Goere D, Cherqui D, Fagniez PL. Surgical treatment of non-colorectal and non-endocrine liver metastases. Gastroenterol Clin Biol. 2000; 24:1177–1185.10. Pang YY. The Brisbane 2000 terminology of liver anatomy and resections. HPB 2000; 2:333-39. HPB (Oxford). 2002; 4:99. author reply 99-100.
Article11. Marudanayagam R, Sandhu B, Perera MT, Taniere P, Coldham C, Bramhall S, et al. Hepatic resection for non-colorectal, non-neuroendocrine, non-sarcoma metastasis: a single-centre experience. HPB (Oxford). 2011; 13:286–292.
Article12. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007; 57:43–66.
Article13. Zinser JW, Hortobagyi GN, Buzdar AU, Smith TL, Fraschini G. Clinical course of breast cancer patients with liver metastases. J Clin Oncol. 1987; 5:773–782.
Article14. Insa A, Lluch A, Prosper F, Marugan I, Martinez-Agullo A, Garcia-Conde J. Prognostic factors predicting survival from first recurrence in patients with metastatic breast cancer: analysis of 439 patients. Breast Cancer Res Treat. 1999; 56:67–78.
Article15. Elias D, Maisonnette F, Druet-Cabanac M, Ouellet JF, Guinebretiere JM, Spielmann M, et al. An attempt to clarify indications for hepatectomy for liver metastases from breast cancer. Am J Surg. 2003; 185:158–164.
Article16. Sakamoto Y, Yamamoto J, Yoshimoto M, Kasumi F, Kosuge T, Kokudo N, et al. Hepatic resection for metastatic breast cancer: prognostic analysis of 34 patients. World J Surg. 2005; 29:524–527.
Article17. Adam R, Aloia T, Krissat J, Bralet MP, Paule B, Giacchetti S, et al. Is liver resection justified for patients with hepatic metastases from breast cancer? Ann Surg. 2006; 244:897–907.
Article18. Adam R, Chiche L, Aloia T, Elias D, Salmon R, Rivoire M, et al. Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann Surg. 2006; 244:524–535.19. Cassera MA, Hammill CW, Ujiki MB, Wolf RF, Swanström LL, Hansen PD. Surgical management of breast cancer liver metastases. HPB (Oxford). 2011; 13:272–278.
Article20. Abbott DE, Brouquet A, Mittendorf EA, Andreou A, Meric-Bernstam F, Valero V, et al. Resection of liver metastases from breast cancer: estrogen receptor status and response to chemotherapy before metastasectomy define outcome. Surgery. 2012; 151:710–716.
Article21. Earle SA, Perez EA, Gutierrez JC, Sleeman D, Livingstone AS, Franceschi D, et al. Hepatectomy enables prolonged survival in select patients with isolated noncolorectal liver metastasis. J Am Coll Surg. 2006; 203:436–446.
Article22. Thelen A, Benckert C, Jonas S, Lopez-Hänninen E, Sehouli J, Neumann U, et al. Liver resection for metastases from breast cancer. J Surg Oncol. 2008; 97:25–29.
Article23. Martinez SR, Young SE, Giuliano AE, Bilchik AJ. The utility of estrogen receptor, progesterone receptor, and Her-2/neu status to predict survival in patients undergoing hepatic resection for breast cancer metastases. Am J Surg. 2006; 191:281–283.
Article24. Cady B, Stone MD, McDermott WV Jr, Jenkins RL, Bothe A Jr, Lavin PT, et al. Technical and biological factors in disease-free survival after hepatic resection for colorectal cancer metastases. Arch Surg. 1992; 127:561–568.
Article25. Gunabushanam G, Sharma S, Thulkar S, Srivastava DN, Rath GK, Julka PK, et al. Radiofrequency ablation of liver metastases from breast cancer: results in 14 patients. J Vasc Interv Radiol. 2007; 18(1 Pt 1):67–72.
Article26. Vogl TJ, Farshid P, Naguib NN, Zangos S. Thermal ablation therapies in patients with breast cancer liver metastases: a review. Eur Radiol. 2013; 23:797–804.
Article27. Cianni R, Pelle G, Notarianni E, Saltarelli A, Rabuffi P, Bagni O, et al. Radioembolisation with (90)Y-labelled resin microspheres in the treatment of liver metastasis from breast cancer. Eur Radiol. 2013; 23:182–189.
Article28. Collettini F, Golenia M, Schnapauff D, Poellinger A, Denecke T, Wust P, et al. Percutaneous computed tomography-guided high-dose-rate brachytherapy ablation of breast cancer liver metastases: initial experience with 80 lesions. J Vasc Interv Radiol. 2012; 23:618–626.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Outcome of Surgical Excision for Isolated Locoregional Recurrence of Breast Cancer
- Surgical Management of Colorectal Liver Metastases
- Prognostic Factors after Hepatic Resection for Metastatic Colorectal Cancer
- Outcome of Surgical Excision for Isolated Locoregional Recurrence of Breast Cancer
- Predictive Factors and Survival Rate for Brain Metastasis from Breast Cancer