J Breast Cancer.
2013 Sep;16(3):315-321. 10.4048/jbc.2013.16.3.315.
The Comparative Study of Ultrasonography, Contrast-Enhanced MRI, and 18F-FDG PET/CT for Detecting Axillary Lymph Node Metastasis in T1 Breast Cancer
- Affiliations
-
- 1Department of Surgery, Kyungpook National University School of Medicine, Daegu, Korea. jjh01@knu.ac.kr
- 2Department of Nuclear Medicine, Kyungpook National University School of Medicine, Daegu, Korea.
- 3Department of Radiology, Kyungpook National University School of Medicine, Daegu, Korea.
- KMID: 2286383
- DOI: http://doi.org/10.4048/jbc.2013.16.3.315
Abstract
- PURPOSE
A more noninvasive evaluation of axillary lymph node in breast cancer is one of the principal challenges of breast cancer treatment. To detect axillary lymph node metastasis (ALNM) in T1 breast cancer, we have compared the axillary ultrasonography (AUS), contrast-enhanced magnetic resonance imaging (cMRI), and 18F-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) to determine the most adequate test or a combination of tests.
METHODS
Retrospectively, 349 T1 breast cancer patients who were preoperatively examined using AUS, cMRI, and PET/CT between 2008 and 2011 and whom underwent pathological evaluations of axillary lymph nodes were reviewed and analyzed.
RESULTS
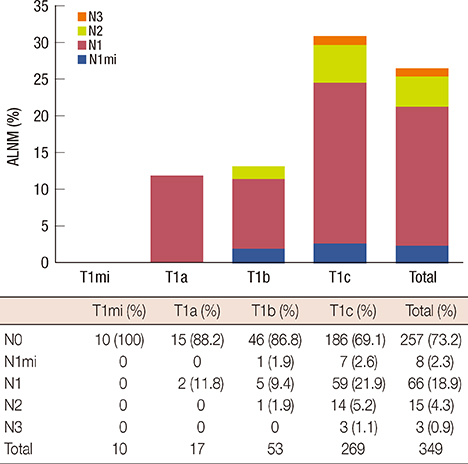
In total, 26.4% (92/349) of patients exhibited ALNM. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of AUS for determining ALNM were 44.6%, 88.7%, 58.6%, 81.7%, and 77.1%, respectively. cMRI was similar to AUS. The sensitivity, specificity, PPV, NPV, and accuracy of PET/CT were 44.5%, 94.2%, 73.2%, 82.6%, and 81.1%, respectively. The combination including cMRI and PET/CT was the most accurate with sensitivity, specificity, PPV, NPV, and accuracy values of 39.1%, 98.8%, 92.3%, 81.9%, and 83.1%, respectively. The mean number (3.5+/-4.2) of ALNMs in the patients who were positive based on cMRI and PET/CT and also pathologically proven to exhibit ALNM was significantly larger than the number (2.16+/-2.26) in other patients who exhibited ALNM (p=0.035).
CONCLUSION
There are no definitive modalities for detecting ALNM in T1 breast cancers to replace sentinel lymph node biopsy (SLNB). If ALNM is suspected based on cMRI and PET/CT, the axillary dissection without SLNB might be a better option because it is related to high possibilities of ALNM and large axillary metastatic volumes.
Keyword
MeSH Terms
Figure
Reference
-
1. Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989; 63:181–187.
Article2. Wilking N, Rutqvist LE, Carstensen J, Mattsson A, Skoog L. Stockholm Breast Cancer Study Group. Prognostic significance of axillary nodal status in primary breast cancer in relation to the number of resected nodes. Acta Oncol. 1992; 31:29–35.
Article3. Giuliano AE, Jones RC, Brennan M, Statman R. Sentinel lymphadenectomy in breast cancer. J Clin Oncol. 1997; 15:2345–2350.
Article4. Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Ashikaga T, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007; 8:881–888.
Article5. Crane-Okada R, Wascher RA, Elashoff D, Giuliano AE. Long-term morbidity of sentinel node biopsy versus complete axillary dissection for unilateral breast cancer. Ann Surg Oncol. 2008; 15:1996–2005.
Article6. Purushotham AD, Upponi S, Klevesath MB, Bobrow L, Millar K, Myles JP, et al. Morbidity after sentinel lymph node biopsy in primary breast cancer: results from a randomized controlled trial. J Clin Oncol. 2005; 23:4312–4321.
Article7. Goyal A, Newcombe RG, Chhabra A, Mansel RE. Morbidity in breast cancer patients with sentinel node metastases undergoing delayed axillary lymph node dissection (ALND) compared with immediate ALND. Ann Surg Oncol. 2008; 15:262–267.
Article8. Rajesh YS, Ellenbogen S, Banerjee B. Preoperative axillary ultrasound scan: its accuracy in assessing the axillary nodal status in carcinoma breast. Breast. 2002; 11:49–52.
Article9. Alvarez S, Añorbe E, Alcorta P, López F, Alonso I, Cortés J. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: a systematic review. AJR Am J Roentgenol. 2006; 186:1342–1348.
Article10. van Rijk MC, Teertstra HJ, Peterse JL, Nieweg OE, Olmos RA, Hoefnagel CA, et al. Ultrasonography and fine-needle aspiration cytology in the preoperative evaluation of melanoma patients eligible for sentinel node biopsy. Ann Surg Oncol. 2006; 13:1511–1516.
Article11. Jung J, Park H, Park J, Kim H. Accuracy of preoperative ultrasound and ultrasound-guided fine needle aspiration cytology for axillary staging in breast cancer. ANZ J Surg. 2010; 80:271–275.
Article12. Bevilacqua JL, Kattan MW, Fey JV, Cody HS 3rd, Borgen PI, Van Zee KJ. Doctor, what are my chances of having a positive sentinel node? A validated nomogram for risk estimation. J Clin Oncol. 2007; 25:3670–3679.
Article13. Viale G, Zurrida S, Maiorano E, Mazzarol G, Pruneri G, Paganelli G, et al. Predicting the status of axillary sentinel lymph nodes in 4351 patients with invasive breast carcinoma treated in a single institution. Cancer. 2005; 103:492–500.
Article14. Houssami N, Ciatto S, Turner RM, Cody HS 3rd, Macaskill P. Preoperative ultrasound-guided needle biopsy of axillary nodes in invasive breast cancer: meta-analysis of its accuracy and utility in staging the axilla. Ann Surg. 2011; 254:243–251.
Article15. Solon JG, Power C, Al-Azawi D, Duke D, Hill AD. Ultrasound-guided core biopsy: an effective method of detecting axillary nodal metastases. J Am Coll Surg. 2012; 214:12–17.
Article16. Mameri CS, Kemp C, Goldman SM, Sobral LA, Ajzen S. Impact of breast MRI on surgical treatment, axillary approach, and systemic therapy for breast cancer. Breast J. 2008; 14:236–244.
Article17. Danforth DN Jr, Aloj L, Carrasquillo JA, Bacharach SL, Chow C, Zujewski J, et al. The role of 18F-FDG-PET in the local/regional evaluation of women with breast cancer. Breast Cancer Res Treat. 2002; 75:135–146.
Article18. Kvistad KA, Rydland J, Smethurst HB, Lundgren S, Fjøsne HE, Haraldseth O. Axillary lymph node metastases in breast cancer: preoperative detection with dynamic contrast-enhanced MRI. Eur Radiol. 2000; 10:1464–1471.
Article19. Peare R, Staff RT, Heys SD. The use of FDG-PET in assessing axillary lymph node status in breast cancer: a systematic review and meta-analysis of the literature. Breast Cancer Res Treat. 2010; 123:281–290.
Article20. Gil-Rendo A, Zornoza G, García-Velloso MJ, Regueira FM, Beorlegui C, Cervera M. Fluorodeoxyglucose positron emission tomography with sentinel lymph node biopsy for evaluation of axillary involvement in breast cancer. Br J Surg. 2006; 93:707–712.
Article21. Luciani A, Dao TH, Lapeyre M, Schwarzinger M, Debaecque C, Lantieri L, et al. Simultaneous bilateral breast and high-resolution axillary MRI of patients with breast cancer: preliminary results. AJR Am J Roentgenol. 2004; 182:1059–1067.
Article22. Valente SA, Levine GM, Silverstein MJ, Rayhanabad JA, Weng-Grumley JG, Ji L, et al. Accuracy of predicting axillary lymph node positivity by physical examination, mammography, ultrasonography, and magnetic resonance imaging. Ann Surg Oncol. 2012; 19:1825–1830.
Article23. García Fernández A, Fraile M, Giménez N, Reñe A, Torras M, Canales L, et al. Use of axillary ultrasound, ultrasound-fine needle aspiration biopsy and magnetic resonance imaging in the preoperative triage of breast cancer patients considered for sentinel node biopsy. Ultrasound Med Biol. 2011; 37:16–22.
Article24. Ko EY, Han BK, Shin JH, Kang SS. Breast MRI for evaluating patients with metastatic axillary lymph node and initially negative mammography and sonography. Korean J Radiol. 2007; 8:382–389.
Article25. Harnan SE, Cooper KL, Meng Y, Ward SE, Fitzgerald P, Papaioannou D, et al. Magnetic resonance for assessment of axillary lymph node status in early breast cancer: a systematic review and meta-analysis. Eur J Surg Oncol. 2011; 37:928–936.
Article26. Memarsadeghi M, Riedl CC, Kaneider A, Galid A, Rudas M, Matzek W, et al. Axillary lymph node metastases in patients with breast carcinomas: assessment with nonenhanced versus uspio-enhanced MR imaging. Radiology. 2006; 241:367–377.
Article27. Veronesi U, De Cicco C, Galimberti VE, Fernandez JR, Rotmensz N, Viale G, et al. A comparative study on the value of FDG-PET and sentinel node biopsy to identify occult axillary metastases. Ann Oncol. 2007; 18:473–478.
Article28. Chae BJ, Bae JS, Kang BJ, Kim SH, Jung SS, Song BJ. Positron emission tomography-computed tomography in the detection of axillary lymph node metastasis in patients with early stage breast cancer. Jpn J Clin Oncol. 2009; 39:284–289.
Article29. Ueda S, Tsuda H, Asakawa H, Omata J, Fukatsu K, Kondo N, et al. Utility of 18F-fluoro-deoxyglucose emission tomography/computed tomography fusion imaging (18F-FDG PET/CT) in combination with ultrasonography for axillary staging in primary breast cancer. BMC Cancer. 2008; 8:165.
Article30. Kim J, Lee J, Chang E, Kim S, Suh K, Sul J, et al. Selective sentinel node plus additional non-sentinel node biopsy based on an FDG-PET/CT scan in early breast cancer patients: single institutional experience. World J Surg. 2009; 33:943–949.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- (18)F-FDG PET/CT with Contrast Enhancement for Evaluation of Axillary Lymph Node Involvement in T1 Breast Cancer
- Supraclavicular Lymph Node Metastasis from Various Malignancies: Assessment with 18F-Fluorodeoxyglucose Positron Emission Tomography/CT, Contrast-Enhanced CT and Ultrasound
- Preoperative Axillary Staging Using 18F-FDG PET/CT and Ultrasonography in Breast Cancer Patients
- 18F-FDG PET/CT Findings in a Breast Cancer Patient with Concomitant Tuberculous Axillary Lymphadenitis
- Prediction of Axillary Nodal Status according to the Axillary Lymph Node to Primary Breast Tumor Maximum Standardized Uptake Value Ratio on 18F-fluorodeoxyglucose Positron Emission Tomography/Computed Tomography