Korean J Physiol Pharmacol.
2015 Sep;19(5):389-399. 10.4196/kjpp.2015.19.5.389.
The Critical Roles of Zinc: Beyond Impact on Myocardial Signaling
- Affiliations
-
- 1Department of Integrated Biomedical Science, Cardiovascular and Metabolic disease Center, College of Medicine, Inje University, Busan 614-735, Korea.
- 2Department of Physiology, Graduate School of Inje University, Cardiovascular and Metabolic Disease Center, Inje University, Busan 614-735, Korea.
- 3College of Medicine, Cardiovascular and Metabolic Disease Center, Inje University, Busan 614-735, Korea. phyhanj@inje.ac.kr
- 4Department of Physiology and Pathophysiology, Tianjin Medical University, Tainjin 300070, P.R. China.
- 5Soonchunhyang Institute of Medio-bio Science (SIMS), Soonchunhyang University, Cheonan 336-745, Korea.
- 6Department of Life Science, Gachon University, Seongnam 461-701, Korea.
- 7Department of Herbal Medicine Resource, Kangwon National University, Samcheok 245-711, Korea.
- KMID: 2285583
- DOI: http://doi.org/10.4196/kjpp.2015.19.5.389
Abstract
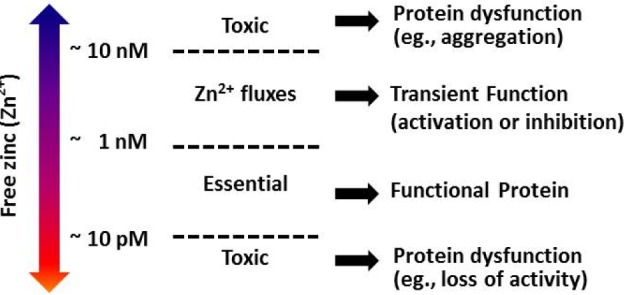
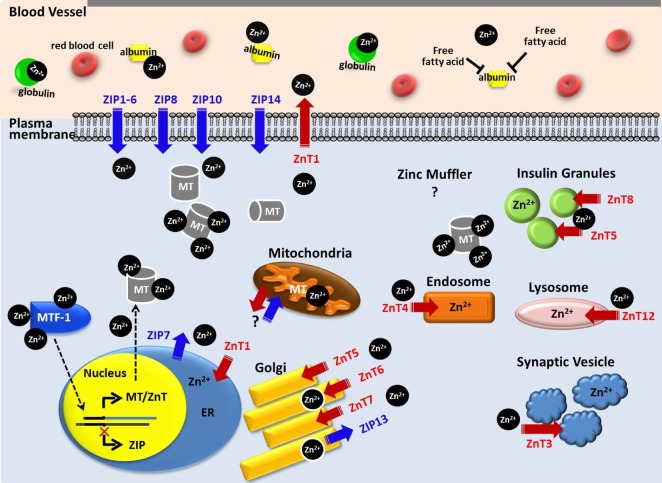
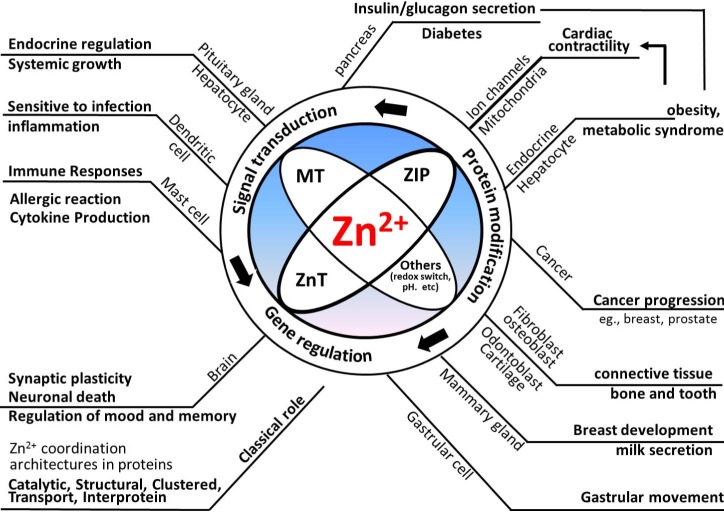
- Zinc has been considered as a vital constituent of proteins, including enzymes. Mobile reactive zinc (Zn2+) is the key form of zinc involved in signal transductions, which are mainly driven by its binding to proteins or the release of zinc from proteins, possibly via a redox switch. There has been growing evidence of zinc's critical role in cell signaling, due to its flexible coordination geometry and rapid shifts in protein conformation to perform biological reactions. The importance and complexity of Zn2+ activity has been presumed to parallel the degree of calcium's participation in cellular processes. Whole body and cellular Zn2+ levels are largely regulated by metallothioneins (MTs), Zn2+ importers (ZIPs), and Zn2+ transporters (ZnTs). Numerous proteins involved in signaling pathways, mitochondrial metabolism, and ion channels that play a pivotal role in controlling cardiac contractility are common targets of Zn2+. However, these regulatory actions of Zn2+ are not limited to the function of the heart, but also extend to numerous other organ systems, such as the central nervous system, immune system, cardiovascular tissue, and secretory glands, such as the pancreas, prostate, and mammary glands. In this review, the regulation of cellular Zn2+ levels, Zn2+-mediated signal transduction, impacts of Zn2+ on ion channels and mitochondrial metabolism, and finally, the implications of Zn2+ in health and disease development were outlined to help widen the current understanding of the versatile and complex roles of Zn2+.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Comparison of electrophysiological effects of calcium channel blockers on cardiac repolarization
Hyang-Ae Lee, Sung-Ae Hyun, Sung-Gurl Park, Ki-Suk Kim, Sung Joon Kim
Korean J Physiol Pharmacol. 2016;20(1):119-127. doi: 10.4196/kjpp.2016.20.1.119.
Reference
-
1. Raulin J. Annales des sciences naturelles. Botanique Et BIologie Vegetale. 1869; 11:93–345.2. Prasad AS, Halsted JA, Nadimi M. Syndrome of iron deficiency anemia, hepatosplenomegaly, hypogonadism, dwarfism and geophagia. Am J Med. 1961; 31:532–546. PMID: 14488490.
Article3. Maret W, Sandstead HH. Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol. 2006; 20:3–18. PMID: 16632171.
Article4. Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993; 73:79–118. PMID: 8419966.
Article5. Andreini C, Bertini I, Rosato A. Metalloproteomes: a bioinformatic approach. Acc Chem Res. 2009; 42:1471–1479. PMID: 19697929.
Article6. Costello LC, Fenselau CC, Franklin RB. Evidence for operation of the direct zinc ligand exchange mechanism for trafficking, transport, and reactivity of zinc in mammalian cells. J Inorg Biochem. 2011; 105:589–599. PMID: 21440525.
Article7. Dudev T, Lim C. Principles governing Mg, Ca, and Zn binding and selectivity in proteins. Chem Rev. 2003; 103:773–788. PMID: 12630852.
Article8. Permyakov EA, Kretsinger RH. Cell signaling, beyond cytosolic calcium in eukaryotes. J Inorg Biochem. 2009; 103:77–86. PMID: 18954910.
Article9. Kochańczyk T, Drozd A, Krężel A. Relationship between the architecture of zinc coordination and zinc binding affinity in proteins--insights into zinc regulation. Metallomics. 2015; 7:244–257. PMID: 25255078.10. Xu Z, Zhou J. Zinc and myocardial ischemia/reperfusion injury. Biometals. 2013; 26:863–878. PMID: 23982700.
Article11. Khan MU, Cheema Y, Shahbaz AU, Ahokas RA, Sun Y, Gerling IC, Bhattacharya SK, Weber KT. Mitochondria play a central role in nonischemic cardiomyocyte necrosis: common to acute and chronic stressor states. Pflugers Arch. 2012; 464:123–131. PMID: 22328074.
Article12. Fukada T, Yamasaki S, Nishida K, Murakami M, Hirano T. Zinc homeostasis and signaling in health and diseases: Zinc signaling. J Biol Inorg Chem. 2011; 16:1123–1134. PMID: 21660546.13. Oteiza PI. Zinc and the modulation of redox homeostasis. Free Radic Biol Med. 2012; 53:1748–1759. PMID: 22960578.
Article14. Hughes S, Samman S. The effect of zinc supplementation in humans on plasma lipids, antioxidant status and thrombogenesis. J Am Coll Nutr. 2006; 25:285–291. PMID: 16943449.
Article15. Taylor KM, Hiscox S, Nicholson RI, Hogstrand C, Kille P. Protein kinase CK2 triggers cytosolic zinc signaling pathways by phosphorylation of zinc channel ZIP7. Sci Signal. 2012; 5:ra11.
Article16. Haase H, Rink L. Zinc signals and immune function. Biofactors. 2014; 40:27–40. PMID: 23804522.
Article17. Takeda A, Nakamura M, Fujii H, Tamano H. Synaptic Zn2+ homeostasis and its significance. Metallomics. 2013; 5:417–423. PMID: 23423555.18. Maret W. Zinc biochemistry: from a single zinc enzyme to a key element of life. Adv Nutr. 2013; 4:82–91. PMID: 23319127.
Article19. Cousins RJ. Absorption, transport, and hepatic metabolism of copper and zinc: special reference to metallothionein and ceruloplasmin. Physiol Rev. 1985; 65:238–309. PMID: 3885271.
Article20. Tran CD, Butler RN, Philcox JC, Rofe AM, Howarth GS, Coyle P. Regional distribution of metallothionein and zinc in the mouse gut: comparison with metallothionien-null mice. Biol Trace Elem Res. 1998; 63:239–251. PMID: 9840820.21. Barnett JP, Blindauer CA, Kassaar O, Khazaipoul S, Martin EM, Sadler PJ, Stewart AJ. Allosteric modulation of zinc speciation by fatty acids. Biochim Biophys Acta. 2013; 1830:5456–5464. PMID: 23726993.
Article22. Scott BJ, Bradwell AR. Identification of the serum binding proteins for iron, zinc, cadmium, nickel, and calcium. Clin Chem. 1983; 29:629–633. PMID: 6831689.
Article23. Taylor KM, Nicholson RI. The LZT proteins; the LIV-1 subfamily of zinc transporters. Biochim Biophys Acta. 2003; 1611:16–30. PMID: 12659941.
Article24. Taylor KM, Morgan HE, Johnson A, Nicholson RI. Structure-function analysis of a novel member of the LIV-1 subfamily of zinc transporters, ZIP14. FEBS Lett. 2005; 579:427–432. PMID: 15642354.
Article25. Sekler I, Sensi SL, Hershfinkel M, Silverman WF. Mechanism and regulation of cellular zinc transport. Mol Med. 2007; 13:337–343. PMID: 17622322.
Article26. Palmiter RD, Huang L. Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. Pflugers Arch. 2004; 447:744–751. PMID: 12748859.
Article27. Powell SR, Aiuto L, Hall D, Tortolani AJ. Zinc supplementation enhances the effectiveness of St. Thomas' Hospital No. 2 cardioplegic solution in an in vitro model of hypothermiccardiac arrest. J Thorac Cardiovasc Surg. 1995; 110:1642–1642. PMID: 8523874.28. Taylor KM, Morgan HE, Johnson A, Nicholson RI. Structure-function analysis of HKE4, a member of the new LIV-1 subfamily of zinc transporters. Biochem J. 2004; 377:131–139. PMID: 14525538.
Article29. Little PJ, Bhattacharya R, Moreyra AE, Korichneva IL. Zinc and cardiovascular disease. Nutrition. 2010; 26:1050–1057. PMID: 20950764.
Article30. Liuzzi JP, Cousins RJ. Mammalian zinc transporters. Annu Rev Nutr. 2004; 24:151–172. PMID: 15189117.
Article31. Carpenè E, Andreani G, Isani G. Metallothionein functions and structural characteristics. J Trace Elem Med Biol. 2007; 21(Suppl 1):35–39. PMID: 18039494.
Article32. Coyle P, Philcox JC, Carey LC, Rofe AM. Metallothionein: the multipurpose protein. Cell Mol Life Sci. 2002; 59:627–647. PMID: 12022471.
Article33. Langmade SJ, Ravindra R, Daniels PJ, Andrews GK. The transcription factor MTF-1 mediates metal regulation of the mouse ZnT1 gene. J Biol Chem. 2000; 275:34803–34809. PMID: 10952993.
Article34. Masters BA, Quaife CJ, Erickson JC, Kelly EJ, Froelick GJ, Zambrowicz BP, Brinster RL, Palmiter RD. Metallothionein III is expressed in neurons that sequester zinc in synaptic vesicles. J Neurosci. 1994; 14:5844–5857. PMID: 7931547.
Article35. Colvin RA, Bush AI, Volitakis I, Fontaine CP, Thomas D, Kikuchi K, Holmes WR. Insights into Zn2+ homeostasis in neurons from experimental and modeling studies. Am J Physiol Cell Physiol. 2008; 294:C726–C742. PMID: 18184873.36. Hogstrand C, Kille P, Nicholson RI, Taylor KM. Zinc transporters and cancer: a potential role for ZIP7 as a hub for tyrosine kinase activation. Trends Mol Med. 2009; 15:101–111. PMID: 19246244.
Article37. Kiedrowski L. Proton-dependent zinc release from intracellular ligands. J Neurochem. 2014; 130:87–96. PMID: 24606401.
Article38. Shuttleworth CW, Weiss JH. Zinc: new clues to diverse roles in brain ischemia. Trends Pharmacol Sci. 2011; 32:480–486. PMID: 21621864.
Article39. Nowakowski A, Petering D. Sensor specific imaging of proteomic Zn2+ with zinquin and TSQ after cellular exposure to N-ethylmaleimide. Metallomics. 2012; 4:448–456. PMID: 22498931.40. Sensi SL, Ton-That D, Sullivan PG, Jonas EA, Gee KR, Kaczmarek LK, Weiss JH. Modulation of mitochondrial function by endogenous Zn2+ pools. Proc Natl Acad Sci U S A. 2003; 100:6157–6162. PMID: 12724524.41. Ohana E, Hoch E, Keasar C, Kambe T, Yifrach O, Hershfinkel M, Sekler I. Identification of the Zn2+ binding site and mode of operation of a mammalian Zn2+ transporter. J Biol Chem. 2009; 284:17677–17686. PMID: 19366695.42. Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002; 109:817–826. PMID: 11901190.
Article43. Maret W. The function of zinc metallothionein: a link between cellular zinc and redox state. J Nutr. 2000; 130(5S Suppl):1455S–1458S. PMID: 10801959.
Article44. Kamalov G, Deshmukh PA, Baburyan NY, Gandhi MS, Johnson PL, Ahokas RA, Bhattacharya SK, Sun Y, Gerling IC, Weber KT. Coupled calcium and zinc dyshomeostasis and oxidative stress in cardiac myocytes and mitochondria of rats with chronic aldosteronism. J Cardiovasc Pharmacol. 2009; 53:414–423. PMID: 19333130.45. Tuncay E, Okatan EN, Toy A, Turan B. Enhancement of cellular antioxidant-defence preserves diastolic dysfunction via regulation of both diastolic Zn2+ and Ca2+ and prevention of RyR2-leak in hyperglycemic cardiomyocytes. Oxid Med Cell Longev. 2014; 2014:290381. PMID: 24693334.46. Klein C, Sunahara RK, Hudson TY, Heyduk T, Howlett AC. Zinc inhibition of cAMP signaling. J Biol Chem. 2002; 277:11859–11865. PMID: 11805091.
Article47. Alvarez-Collazo J, Diaz-Garcia CM, Lopez-Medina AI, Vassort G, Alvarez JL. Zinc modulation of basal and β-adrenergically stimulated L-type Ca2+ current in rat ventricular cardiomyocytes: consequences in cardiac diseases. Pflugers Arch. 2012; 464:459–470. PMID: 23007464.48. Pucéat M, Bony C, Jaconi M, Vassort G. Specific activation of adenylyl cyclase V by a purinergic agonist. FEBS Lett. 1998; 431:189–194. PMID: 9708900.
Article49. Gómez AM, Kerfant BG, Vassort G. Microtubule disruption modulates Ca2+ signaling in rat cardiac myocytes. Circ Res. 2000; 86:30–36. PMID: 10625302.50. Volpe SL, Lowe NM, Woodhouse LR, King JC. Effect of maximal exercise on the short-term kinetics of zinc metabolism in sedentary men. Br J Sports Med. 2007; 41:156–161. PMID: 17138634.
Article51. Bellomo E, Massarotti A, Hogstrand C, Maret W. Zinc ions modulate protein tyrosine phosphatase 1B activity. Metallomics. 2014; 6:1229–1239. PMID: 24793162.
Article52. Pandey NR, Vardatsikos G, Mehdi MZ, Srivastava AK. Cell-type-specific roles of IGF-1R and EGFR in mediating Zn2+-induced ERK1/2 and PKB phosphorylation. J Biol Inorg Chem. 2010; 15:399–407. PMID: 19946718.53. Haase H, Maret W. Protein tyrosine phosphatases as targets of the combined insulinomimetic effects of zinc and oxidants. Biometals. 2005; 18:333–338. PMID: 16158225.
Article54. Aslund F, Beckwith J. Bridge over troubled waters: sensing stress by disulfide bond formation. Cell. 1999; 96:751–753. PMID: 10102262.55. Turan B, Fliss H, Desilets M. Oxidants increase intracellular free Zn2+ concentration in rabbit ventricular myocytes. Am J Physiol. 1997; 272:H2095–H2106. PMID: 9176274.56. Tuncay E, Bilginoglu A, Sozmen NN, Zeydanli EN, Ugur M, Vassort G, Turan B. Intracellular free zinc during cardiac excitation-contraction cycle: calcium and redox dependencies. Cardiovasc Res. 2011; 89:634–642. PMID: 21062918.
Article57. Tuncay E, Okatan EN, Vassort G, Turan B. β-blocker timolol prevents arrhythmogenic Ca2+ release and normalizes Ca2+ and Zn2+ dyshomeostasis in hyperglycemic rat heart. PLoS One. 2013; 8:e71014. PMID: 23923043.58. Korichneva I, Hoyos B, Chua R, Levi E, Hammerling U. Zinc release from protein kinase C as the common event during activation by lipid second messenger or reactive oxygen. J Biol Chem. 2002; 277:44327–44331. PMID: 12213816.
Article59. Wang G, Strang C, Pfaffinger PJ, Covarrubias M. Zn2+-dependent redox switch in the intracellular T1-T1 interface of a Kv channel. J Biol Chem. 2007; 282:13637–13647. PMID: 17331952.60. Zhang S, Kehl SJ, Fedida D. Modulation of Kv1.5 potassium channel gating by extracellular zinc. Biophys J. 2001; 81:125–136. PMID: 11423401.
Article61. Mahaut-Smith MP. The effect of zinc on calcium and hydrogen ion currents in intact snail neurones. J Exp Biol. 1989; 145:455–464. PMID: 22912993.
Article62. Stanfield PR. The effect of zinc ions on the gating of the delayed potassium conductance of frog sartorius muscle. J Physiol. 1975; 251:711–735. PMID: 1081141.
Article63. Vogt K, Mellor J, Tong G, Nicoll R. The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron. 2000; 26:187–196. PMID: 10798403.
Article64. Kerchner GA, Canzoniero LM, Yu SP, Ling C, Choi DW. Zn2+ current is mediated by voltage-gated Ca2+ channels and enhanced by extracellular acidity in mouse cortical neurones. J Physiol. 2000; 528 Pt 1:39–52. PMID: 11018104.65. Atar D, Backx PH, Appel MM, Gao WD, Marban E. Excitation-transcription coupling mediated by zinc influx through voltage-dependent calcium channels. J Biol Chem. 1995; 270:2473–2477. PMID: 7852308.
Article66. Andersson DA, Gentry C, Moss S, Bevan S. Clioquinol and pyrithione activate TRPA1 by increasing intracellular Zn2+. Proc Natl Acad Sci U S A. 2009; 106:8374–8379. PMID: 19416844.67. Hu H, Bandell M, Petrus MJ, Zhu MX, Patapoutian A. Zinc activates damage-sensing TRPA1 ion channels. Nat Chem Biol. 2009; 5:183–190. PMID: 19202543.
Article68. Dineley KE, Votyakova TV, Reynolds IJ. Zinc inhibition of cellular energy production: implications for mitochondria and neurodegeneration. J Neurochem. 2003; 85:563–570. PMID: 12694382.
Article69. Ye B, Maret W, Vallee BL. Zinc metallothionein imported into liver mitochondria modulates respiration. Proc Natl Acad Sci U S A. 2001; 98:2317–2322. PMID: 11226237.
Article70. Kleiner D. The effect of Zn2+ ions on mitochondrial electron transport. Arch Biochem Biophys. 1974; 165:121–125. PMID: 4374126.71. Maret W, Jacob C, Vallee BL, Fischer EH. Inhibitory sites in enzymes: zinc removal and reactivation by thionein. Proc Natl Acad Sci U S A. 1999; 96:1936–1940. PMID: 10051573.
Article72. Meeusen JW, Nowakowski A, Petering DH. Reaction of metal-binding ligands with the zinc proteome: zinc sensors and N,N,N',N'-tetrakis(2-pyridylmethyl)ethylenediamine. Inorg Chem. 2012; 51:3625–3632. PMID: 22380934.
Article73. Vallee BL. Metallothionein: historical review and perspectives. Experientia Suppl. 1979; 34:19–39. PMID: 400521.
Article74. Maret W, Heffron G, Hill HA, Djuricic D, Jiang LJ, Vallee BL. The ATP/metallothionein interaction: NMR and STM. Biochemistry. 2002; 41:1689–1694. PMID: 11814364.
Article75. Skulachev VP, Evtodienko IuV, Iasaĭtis AA, Gmirnova EG, Chistiakov VV. Reversible suppression of electron transfer between cytochromes B and C. Vopr Med Khim. 1966; 12:438–440. PMID: 4299500.76. Berry EA, Zhang Z, Bellamy HD, Huang L. Crystallographic location of two Zn2+-binding sites in the avian cytochrome bc(1) complex. Biochim Biophys Acta. 2000; 1459:440–448. PMID: 11004461.77. Brown AM, Kristal BS, Effron MS, Shestopalov AI, Ullucci PA, Sheu KF, Blass JP, Cooper AJ. Zn2+ inhibits alpha-ketoglutarate-stimulated mitochondrial respiration and the isolated alpha-ketoglutarate dehydrogenase complex. J Biol Chem. 2000; 275:13441–13447. PMID: 10788456.78. Gazaryan IG, Krasnikov BF, Ashby GA, Thorneley RN, Kristal BS, Brown AM. Zinc is a potent inhibitor of thiol oxidoreductase activity and stimulates reactive oxygen species production by lipoamide dehydrogenase. J Biol Chem. 2002; 277:10064–10072. PMID: 11744691.
Article79. Feng W, Cai J, Pierce WM, Franklin RB, Maret W, Benz FW, Kang YJ. Metallothionein transfers zinc to mitochondrial aconitase through a direct interaction in mouse hearts. Biochem Biophys Res Commun. 2005; 332:853–858. PMID: 15913554.
Article80. Kelleher SL, McCormick NH, Velasquez V, Lopez V. Zinc in specialized secretory tissues: roles in the pancreas, prostate, and mammary gland. Adv Nutr. 2011; 2:101–111. PMID: 22332039.
Article81. Costello LC, Franklin RB, Feng P. Mitochondrial function, zinc, and intermediary metabolism relationships in normal prostate and prostate cancer. Mitochondrion. 2005; 5:143–153. PMID: 16050980.
Article82. Devinney MJ, Malaiyandi LM, Vergun O, DeFranco DB, Hastings TG, Dineley KE. A comparison of Zn2+- and Ca2+-triggered depolarization of liver mitochondria reveals no evidence of Zn2+-induced permeability transition. Cell Calcium. 2009; 45:447–455. PMID: 19349076.83. Wudarczyk J, Debska G, Lenartowicz E. Zinc as an inducer of the membrane permeability transition in rat liver mitochondria. Arch Biochem Biophys. 1999; 363:1–8. PMID: 10049493.
Article84. Feng P, Li TL, Guan ZX, Franklin RB, Costello LC. Direct effect of zinc on mitochondrial apoptogenesis in prostate cells. Prostate. 2002; 52:311–318. PMID: 12210492.
Article85. Feng P, Li T, Guan Z, Franklin RB, Costello LC. The involvement of Bax in zinc-induced mitochondrial apoptogenesis in malignant prostate cells. Mol Cancer. 2008; 7:25. PMID: 18331646.
Article86. Calderone A, Jover T, Mashiko T, Noh KM, Tanaka H, Bennett MV, Zukin RS. Late calcium EDTA rescues hippocampal CA1 neurons from global ischemia-induced death. J Neurosci. 2004; 24:9903–9913. PMID: 15525775.
Article87. McCord MC, Aizenman E. The role of intracellular zinc release in aging, oxidative stress, and Alzheimer's disease. Front Aging Neurosci. 2014; 6:77. PMID: 24860495.
Article88. Haase H, Rink L. Multiple impacts of zinc on immune function. Metallomics. 2014; 6:1175–1180. PMID: 24531756.
Article90. Frederickson C, Frederickson CJ, Maret W, Sandstead H, Giblin L, Thompson R. Meeting report: zinc signals 2007-expanding roles of the free zinc ion in biology. Sci STKE. 2007; 2007:pe61. PMID: 17986712.
Article91. Foster M, Samman S. Zinc and regulation of inflammatory cytokines: implications for cardiometabolic disease. Nutrients. 2012; 4:676–694. PMID: 22852057.
Article92. Holst B, Egerod KL, Schild E, Vickers SP, Cheetham S, Gerlach LO, Storjohann L, Stidsen CE, Jones R, Beck-Sickinger AG, Schwartz TW. GPR39 signaling is stimulated by zinc ions but not by obestatin. Endocrinology. 2007; 148:13–20. PMID: 16959833.
Article93. Młyniec K, Budziszewska B, Holst B, Ostachowicz B, Nowak G. GPR39 (zinc receptor) knockout mice exhibit depressionlike behavior and CREB/BDNF down-regulation in the hippocampus. Int J Neuropsychopharmacol. 2014; 18:pii: pyu002. DOI: 10.1093/ijnp/pyu002.
Article94. Popovics P, Stewart AJ. GPR39: a Zn2+-activated G protein-coupled receptor that regulates pancreatic, gastrointestinal and neuronal functions. Cell Mol Life Sci. 2011; 68:85–95. PMID: 20812023.95. Halas ES, Hunt CD, Eberhardt MJ. Learning and memory disabilities in young adult rats from mildly zinc deficient dams. Physiol Behav. 1986; 37:451–458. PMID: 3749304.
Article96. Adamo AM, Zago MP, Mackenzie GG, Aimo L, Keen CL, Keenan A, Oteiza PI. The role of zinc in the modulation of neuronal proliferation and apoptosis. Neurotox Res. 2010; 17:1–14. PMID: 19784710.
Article97. Seth R, Corniola RS, Gower-Winter SD, Morgan TJ Jr, Bishop B, Levenson CW. Zinc deficiency induces apoptosis via mitochondrial p53- and caspase-dependent pathways in human neuronal precursor cells. J Trace Elem Med Biol. 2015; 30:59–65. PMID: 25467851.
Article98. Weiss JH, Sensi SL, Koh JY. Zn2+: a novel ionic mediator of neural injury in brain disease. Trends Pharmacol Sci. 2000; 21:395–401. PMID: 11050320.99. Lee J, Kim CH, Kim DG, Ahn YS. Zinc inhibits amyloid beta production from Alzheimer's amyloid precursor protein in SH-SY5Y cells. Korean J Physiol Pharmacol. 2009; 13:195–200. PMID: 19885037.100. Tamaki M, Fujitani Y. Role of zinc in type 2 diabetes. Nihon Eiseigaku Zasshi. 2014; 69:15–23. PMID: 24476591.
Article101. Miao X, Sun W, Fu Y, Miao L, Cai L. Zinc homeostasis in the metabolic syndrome and diabetes. Front Med. 2013; 7:31–52. PMID: 23385610.
Article102. Yi T, Vick JS, Vecchio MJ, Begin KJ, Bell SP, Delay RJ, Palmer BM. Identifying cellular mechanisms of zinc-induced relaxation in isolated cardiomyocytes. Am J Physiol Heart Circ Physiol. 2013; 305:H706–H715. PMID: 23812383.
Article103. Ciofalo FR, Thomas LJ Jr. The effects of zinc on contractility, membrane potentials, and cation content of rat atria. J Gen Physiol. 1965; 48:825–839. PMID: 14324990.
Article104. Schnieden H, Small RC. Spasmolytic effects of cadmium and zinc ions upon the guinea-pig isolated ileum preparation. Br J Pharmacol. 1971; 41:488–499. PMID: 4396970.
Article105. Evangelou A, Kalfakakou V. Electrocardiographic alterations induced by zinc ions on isolated guinea pig heart preparations. Biol Trace Elem Res. 1993; 36:203–208. PMID: 7681312.
Article106. Oster O, Dahm M, Oelert H. Element concentrations (selenium, copper, zinc, iron, magnesium, potassium, phosphorous) in heart tissue of patients with coronary heart disease correlated with physiological parameters of the heart. Eur Heart J. 1993; 14:770–774. PMID: 8325303.
Article107. Shokrzadeh M, Ghaemian A, Salehifar E, Aliakbari S, Saravi SS, Ebrahimi P. Serum zinc and copper levels in ischemic cardiomyopathy. Biol Trace Elem Res. 2009; 127:116–123. PMID: 18953508.
Article108. Witte KK, Clark AL, Cleland JG. Chronic heart failure and micronutrients. J Am Coll Cardiol. 2001; 37:1765–1774. PMID: 11401109.
Article109. Gomez E, del Diego C, Orden I, Elósegui LM, Borque L, Escanero JF. Longitudinal study of serum copper and zinc levels and their distribution in blood proteins after acute myocardial infarction. J Trace Elem Med Biol. 2000; 14:65–70. PMID: 10941714.
Article110. Foster M, Samman S. Zinc and redox signaling: perturbations associated with cardiovascular disease and diabetes mellitus. Antioxid Redox Signal. 2010; 13:1549–1573. PMID: 20568953.
Article111. Cohen N, Golik A. Zinc balance and medications commonly used in the management of heart failure. Heart Fail Rev. 2006; 11:19–24. PMID: 16819574.
Article112. Meerarani P, Reiterer G, Toborek M, Hennig B. Zinc modulates PPARgamma signaling and activation of porcine endothelial cells. J Nutr. 2003; 133:3058–3064. PMID: 14519784.113. Cho YS, Lee KH, Park JW. Pyrithione-zinc Prevents UVB-induced Epidermal Hyperplasia by Inducing HIF-1alpha. Korean J Physiol Pharmacol. 2010; 14:91–97. PMID: 20473380.114. Alcantara EH, Shin MY, Feldmann J, Nixon GF, Beattie JH, Kwun IS. Long-term zinc deprivation accelerates rat vascular smooth muscle cell proliferation involving the down-regulation of JNK1/2 expression in MAPK signaling. Atherosclerosis. 2013; 228:46–52. PMID: 23466072.
Article115. Prost AL, Bloc A, Hussy N, Derand R, Vivaudou M. Zinc is both an intracellular and extracellular regulator of KATP channel function. J Physiol. 2004; 559:157–167. PMID: 15218066.116. Wroblewski N, Schill WB, Henkel R. Metal chelators change the human sperm motility pattern. Fertil Steril. 2003; 79(Suppl 3):1584–1589. PMID: 12801564.
Article117. Costello LC, Franklin RB. Cytotoxic/tumor suppressor role of zinc for the treatment of cancer: an enigma and an opportunity. Expert Rev Anticancer Ther. 2012; 12:121–128. PMID: 22149438.
Article118. Chowanadisai W, Lönnerdal B, Kelleher SL. Identification of a mutation in SLC30A2 (ZnT-2) in women with low milk zinc concentration that results in transient neonatal zinc deficiency. J Biol Chem. 2006; 281:39699–39707. PMID: 17065149.
Article119. Fraker PJ, King LE. Reprogramming of the immune system during zinc deficiency. Annu Rev Nutr. 2004; 24:277–298. PMID: 15189122.
Article120. Donnelly TE Jr. Effects of zinc chloride on the hydrolysis of cyclic GMP and cyclic AMP by the activator-dependent cyclic nucleotide phosphodiesterase from bovine heart. Biochim Biophys Acta. 1978; 522:151–160. PMID: 202321.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endogenous Zinc in Neurological Diseases
- The Zinc Transport Systems and Their Regulation in Pathogenic Fungi
- Acrodermatitis Enteropathica in Two Siblings: treated with zinc sulfate
- A Case of Symptomatic Zinc Deficiency due to Total Parenteral Nutrition
- Zinc upregulates bone-specific transcription factor Runx2 expression via BMP-2 signaling and Smad-1 phosphorylation in osteoblasts