Endocrinol Metab.
2012 Sep;27(3):227-231. 10.3803/EnM.2012.27.3.227.
A Case of Incidental Struma Ovarii Confounded with the Metastasis of Papillary Thyroid Cancer
- Affiliations
-
- 1Department of Internal Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea. drkhs@catholic.ac.kr
- 2Department of Pathology, The Catholic University of Korea College of Medicine, Seoul, Korea.
- KMID: 2282407
- DOI: http://doi.org/10.3803/EnM.2012.27.3.227
Abstract
- A focal radioactive iodine uptake in the pelvis of a patient with differentiated thyroid cancer needs differential diagnosis besides bone metastasis. Struma ovarii is a rare monodermal ovarian teratoma composed predominantly of mature thyroid tissue; 5-10% of these tumors are malignant. As diagnosis and surgery of thyroid cancer have increased recently, incidental cases of struma ovarii, after radioactive iodine treatment, were occasionally reported. Rare cases of ovary metastasis of thyroid cancer were also reported. We report a case of benign struma ovarii incidentally found in a patient with papillary thyroid cancer. The patient showed a sustained high level of thyroglobulin and focal radioactive iodine uptake in the right pelvis, confused with distant metastasis, after total thyroidectomy and radioactive iodine treatment.
MeSH Terms
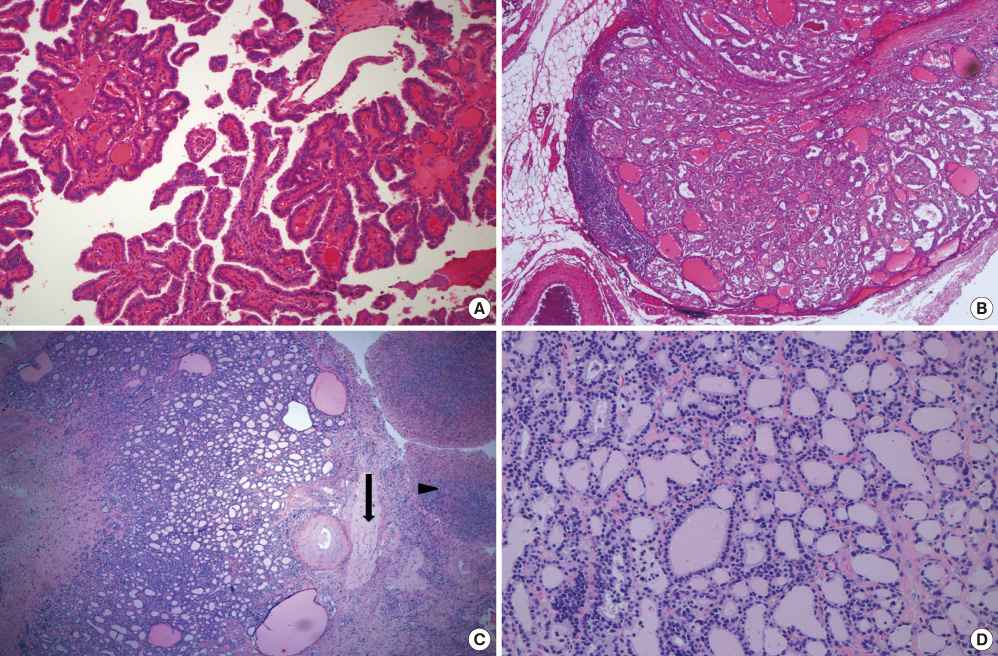
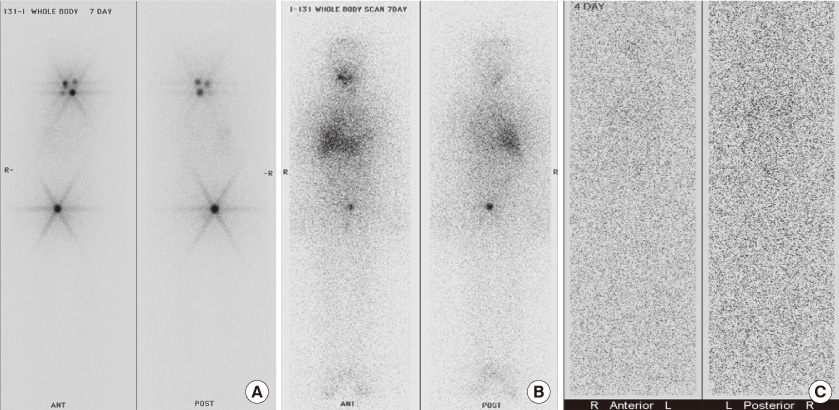
Figure
Reference
-
1. Outwater EK, Siegelman ES, Hunt JL. Ovarian teratomas: tumor types and imaging characteristics. Radiographics. 2001. 21:475–490.2. Yoo SC, Chang KH, Lyu MO, Chang SJ, Ryu HS, Kim HS. Clinical characteristics of struma ovarii. J Gynecol Oncol. 2008. 19:135–138.3. Kim SJ, Pak K, Lim HJ, Yun KH, Seong SJ, Kim TJ, Lim KT, Jung HW, Park IS, Shim JU, Park CT, Lee KH. Clinical diversity of struma ovarii. Korean J Obstet Gynecol. 2002. 45:748–752.4. Salman WD, Singh M, Twaij Z. A case of papillary thyroid carcinoma in struma ovarii and review of the literature. Patholog Res Int. 2010. 2010:352476.5. Ghander C, Lussato D, Conte Devolx B, Mundler O, Taieb D. Incidental diagnosis of struma ovarii after thyroidectomy for thyroid cancer: functional imaging studies and follow-up. Gynecol Oncol. 2006. 102:378–380.6. Lim ST, Jeong HJ, Chung MJ, Yim CY, Sohn MH. Malignant struma ovarii demonstrated on post-therapy radioiodine scan after total thyroidectomy for papillary thyroid cancer. Clin Nucl Med. 2008. 33:429–431.7. Brogioni S, Viacava P, Tomisti L, Martino E, Macchia E. A special case of bilateral ovarian metastases in a woman with papillary carcinoma of the thyroid. Exp Clin Endocrinol Diabetes. 2007. 115:397–400.8. Dardik RB, Dardik M, Westra W, Montz FJ. Malignant struma ovarii: two case reports and a review of the literature. Gynecol Oncol. 1999. 73:447–451.9. Kano H, Inoue M, Nishino T, Yoshimoto Y, Arima R. Malignant struma ovarii with Graves' disease. Gynecol Oncol. 2000. 79:508–510.10. Makani S, Kim W, Gaba AR. Struma Ovarii with a focus of papillary thyroid cancer: a case report and review of the literature. Gynecol Oncol. 2004. 94:835–839.11. Jung SI, Kim YJ, Lee MW, Jeon HJ, Choi JS, Moon MH. Struma ovarii: CT findings. Abdom Imaging. 2008. 33:740–743.12. Matsuki M, Kaji Y, Matsuo M, Kobashi Y. Struma ovarii: MRI findings. Br J Radiol. 2000. 73:87–90.13. Matsuda K, Maehama T, Kanazawa K. Malignant struma ovarii with thyrotoxicosis. Gynecol Oncol. 2001. 82:575–577.14. Rose PG, Arafah B, Abdul-Karim FW. Malignant struma ovarii: recurrence and response to treatment monitored by thyroglobulin levels. Gynecol Oncol. 1998. 70:425–427.15. Hocevar M, Auersperg M, Stanovnik L. The dynamics of serum thyroglobulin elimination from the body after thyroid surgery. Eur J Surg Oncol. 1997. 23:208–210.16. Giovanella L, Ceriani L, Maffioli M. Postsurgery serum thyroglobulin disappearance kinetic in patients with differentiated thyroid carcinoma. Head Neck. 2010. 32:568–571.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Papillary type thyroid carcinoma originating in struma ovarii: Case report and review of the literature
- A Case of Malignant Struma Ovarii with Cervical Papillary Thyroid Carcinoma
- A Case of Struma Ovarii
- Malignant Struma Ovarii With Graves’ Disease and Papillary Thyroid Carcinoma: A Case Report
- Malignant struma ovarii with hyperthyroidism ; radionuclide study and treatment