Ann Dermatol.
2014 Aug;26(4):462-468. 10.5021/ad.2014.26.4.462.
Efficacy and Safety Results of a Drug-Free Cosmetic Fluid for Perioral Dermatitis: The Toleriane Fluide Efficacy in Perioral Dermatitis (TOLPOD) Study
- Affiliations
-
- 1Department of Dermatology and Allergy, Ludwig Maximilian University, Munich, Germany. wollenberg@lrz.uni-muenchen.de
- 2Department of Dermatology, Heinrich Heine University, Dusseldorf, Germany.
- KMID: 2265586
- DOI: http://doi.org/10.5021/ad.2014.26.4.462
Abstract
- BACKGROUND
Perioral dermatitis (POD) is a common inflammatory skin disease without standard therapy.
OBJECTIVE
We sought to evaluate the clinical value of a soothing fluid for the treatment of POD.
METHODS
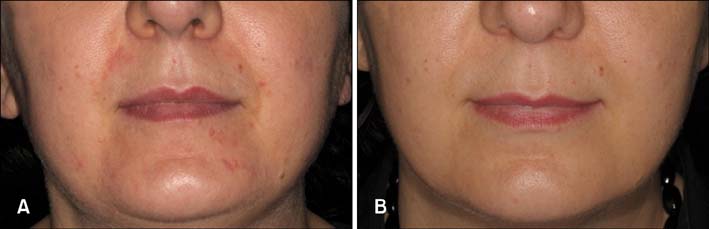
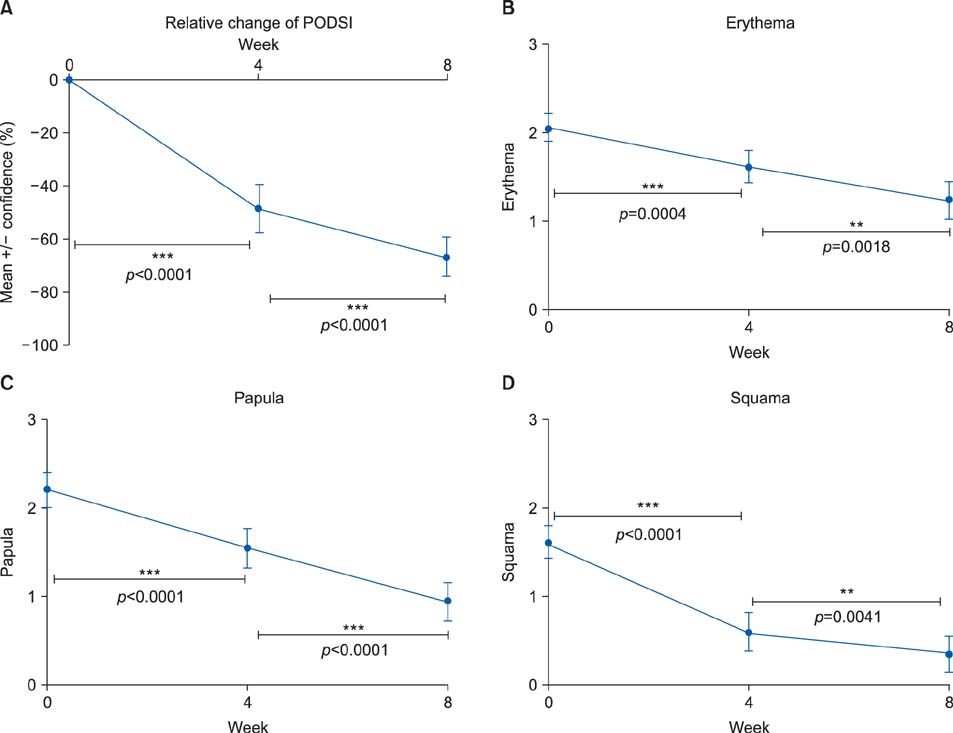
We included 51 patients with POD in this 8-week clinical trial. The Toleriane Fluide Efficacy in Perioral Dermatitis (TOLPOD) study had an open-label design and involved twice-daily application of Toleriane Fluide, a soothing cosmetic fluid. Clinical assessment of POD was performed with a predefined questionnaire including the POD severity index (PODSI). Control visits were made after 4 and 8 weeks of treatment.
RESULTS
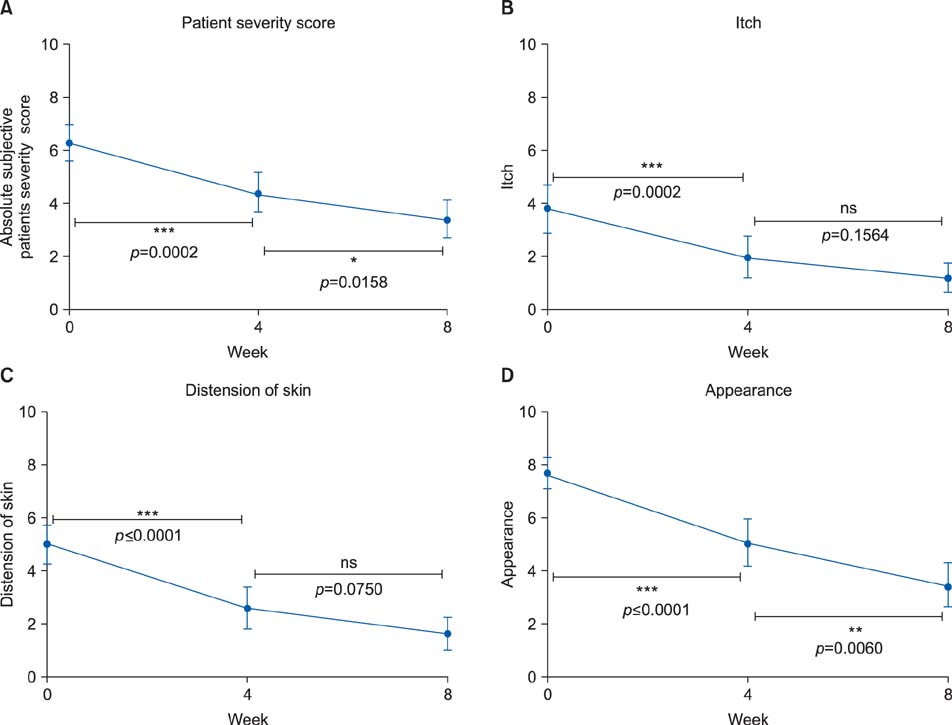
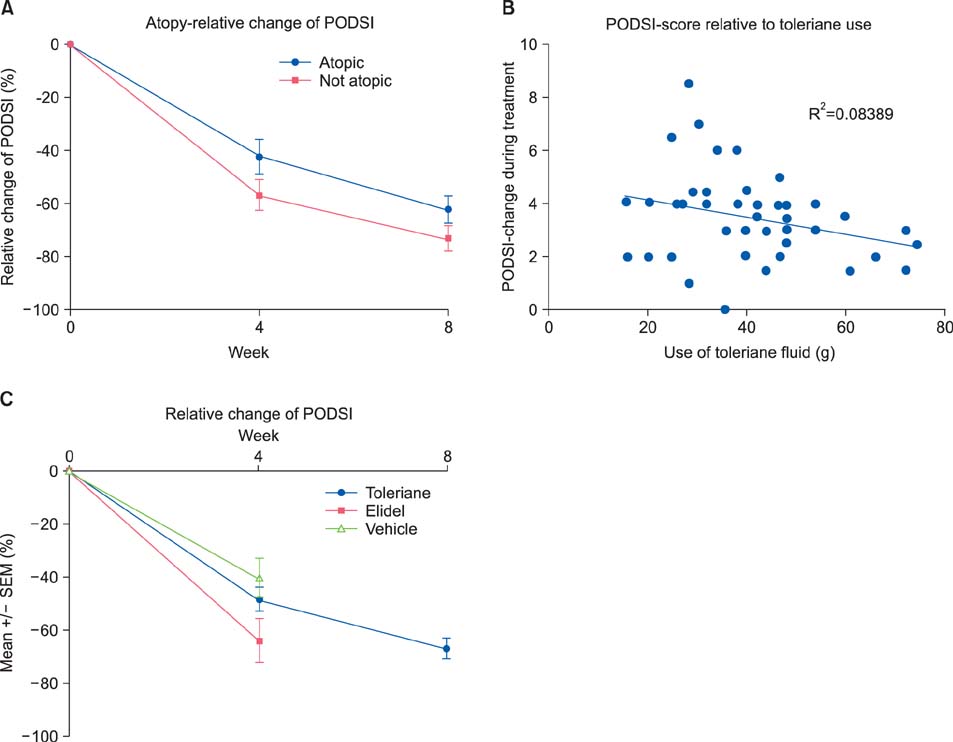
The results were compared with those of a historical control group treated with a vehicle cream. Patients treated with the soothing fluid showed a continuous and significant improvement of the PODSI over time. The improvement of PODSI observed with the soothing fluid was better, but not significantly better, than that observed in the historical controls. In addition, the subjective complaints of patients such as disease burden, itching, distension of the skin, and appearance improved during treatment.
CONCLUSION
A soothing fluid could be a clinically useful treatment option for POD.
Keyword
Figure
Reference
-
1. Mihan R, Ayres S Jr. Perioral dermatitis. Arch Dermatol. 1964; 89:803–805.
Article2. Hafeez ZH. Perioral dermatitis: an update. Int J Dermatol. 2003; 42:514–517.
Article3. Schwarz T, Kreiselmaier I, Bieber T, Thaci D, Simon JC, Meurer M, et al. A randomized, double-blind, vehicle-controlled study of 1% pimecrolimus cream in adult patients with perioral dermatitis. J Am Acad Dermatol. 2008; 59:34–40.
Article4. Dirschka T, Tronnier H, Fölster-Holst R. Epithelial barrier function and atopic diathesis in rosacea and perioral dermatitis. Br J Dermatol. 2004; 150:1136–1141.
Article5. Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol. 2006; 54:1–15.
Article6. Wollenberg A, Bieber T, Dirschka T, Luger T, Meurer M, Proksch E, et al. Perioral dermatitis. J Dtsch Dermatol Ges. 2011; 9:422–427.
Article7. Wollenberg A, Oppel T. Scoring of skin lesions with the perioral dermatitis severity index (PODSI). Acta Derm Venereol. 2006; 86:251–252.
Article8. Oppel T, Pavicic T, Kamann S, Bräutigam M, Wollenberg A. Pimecrolimus cream (1%) efficacy in perioral dermatitis - results of a randomized, double-blind, vehicle-controlled study in 40 patients. J Eur Acad Dermatol Venereol. 2007; 21:1175–1180.
Article9. Malik R, Quirk CJ. Topical applications and perioral dermatitis. Australas J Dermatol. 2000; 41:34–38.
Article10. Weber K, Thurmayr R, Meisinger A. A topical erthromycin preparation and oral tetracycline for the treatment of perioral dermatitis: A placebo-controlled trial. J Dermatol Treat. 1993; 4:57–59.
Article