Korean J Lab Med.
2006 Apr;26(2):98-102. 10.3343/kjlm.2006.26.2.98.
Production of Antibody against Helicobacter pylori HP0231
- Affiliations
-
- 1Department of Laboratory Medicine, College of Medicine, Konyang University, Daejeon, Korea.
- 2Department of Biotechnology, College of Agriculture and Life Science, Hankyong National University, Anseong, Korea.
- 3Department of Food and Nutrition, Seoil College, Seoul, Korea. wjjson@seoil.ac.kr
- KMID: 2238898
- DOI: http://doi.org/10.3343/kjlm.2006.26.2.98
Abstract
-
BACKGROUND: Stool antigen detection kits for diagnosis of infection of Helicobacter pylori have been widely used for their convenience, but are mostly imported. Since Helicobacter pylori strains show a distinctive genetic diversity, it is important to find a protein that is a common antigen among various strains and shows a strong immunogenicity for the development of a stool antigen detection kit. HP0231 protein strongly reacts with the sera of patients suffering from gastritis and peptic ulcer. Therefore, HP0231 is an excellent candidate as a target gene for this study.
METHODS
Chromosomal DNA from H. pylori was isolated. HP0231 gene was amplified by PCR, cloned into pET28a(+) vector, and overexpressed using isopropyl-beta-D-thiogalactopyranoside in E. coli BL21 (DE3). HP0231 protein was purified by Ni-NTA affinity chromatography followed by electroelution after SDS-PAGE. Rabbits were immunized with the purified HP0231 protein for the production of antibodies. Rabbit anti-HP0231 antibody was partially purified and tested for the sensitivity and specificity using ELISA and Western Blot Analysis.
RESULTS
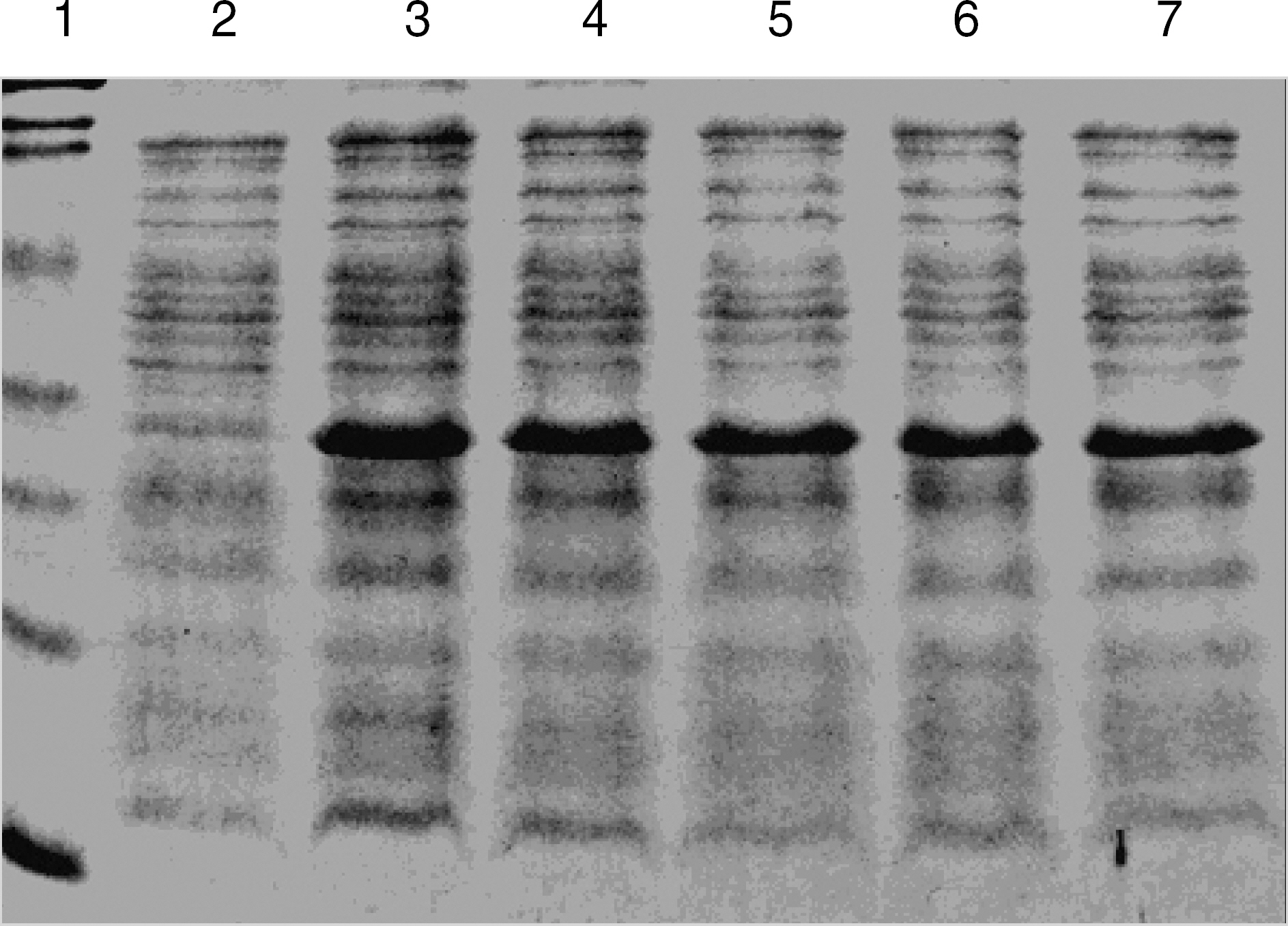
The sequence of the cloned HP0231 gene was identical with the gene sequence from Genbank (AA216016). HP0231 gene was overexpressed and HP0231 protein was purified. Rabbit anti-HP0231 antibody produced after immunization with the purified HP0231 protein reacted with the purified HP0231 protein, cell extracts from cultured H. pylori, and stomach biopsy tissue from patients, but not with cell extracts from cultured E. coli used as a negative control. After 1 million fold dilution, rabbit anti-HP0231 antibody still reacted with 1 microgram of HP0231 protein.
CONCLUSIONS
Rabbit anti-HP0231 antibody was produced to detect HP0231 protein of H. pylori and will be tested for the development of a stool antigen detection kit for H. pylori.
MeSH Terms
-
Antibodies
Biopsy
Blotting, Western
Cell Extracts
Chromatography, Affinity
Clone Cells
Databases, Nucleic Acid
Diagnosis
DNA
Electrophoresis, Polyacrylamide Gel
Enzyme-Linked Immunosorbent Assay
Gastritis
Genetic Variation
Helicobacter pylori*
Helicobacter*
Humans
Immunization
Peptic Ulcer
Polymerase Chain Reaction
Rabbits
Sensitivity and Specificity
Stomach
Antibodies
Cell Extracts
DNA
Figure
Reference
-
References
1. Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984; 1:1311–5.
Article2. Goodwin CS, Armstrong JA, Marshall BJ. Campylobacter pyloridis, gastritis, and peptic ulceration. J Clin Pathol. 1986; 39:353–65.3. Blaser MJ. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990; 161:626–33.4. Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991; 325:1127–31.5. Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994; 330:1267–71.6. Perri F, Quitadamo M, Ricciardi R, Piepoli A, Cotugno R, Gentile A, et al. Comparison of a monoclonal antigen stool test (Hp StAR) with the 13C-urea breath test in monitoring Helicobacter pylori eradication therapy. World J Gastroenterol. 2005; 11:5878–81.7. Nakata H, Itoh H, Ishiguchi T, Iwata T, Sato H, Higashimoto Y, et al. Immunological rapid urease test using monoclonal antibody for Helicobacter pylori. J Gastroenterol Hepatol. 2004; 19:970–4.8. Cover TL, Blaser MJ. Helicobacter pylori infection, a paradigm for chronic mucosal inflammation: pathogenesis and implications for eradication and prevention. Adv Intern Med. 1996; 41:85–117.9. Del Giudice G, Covacci A, Telford JL, Montecucco C, Rappuoli R. The design of vaccines against Helicobacter pylori and their development. Annu Rev Immunol. 2001; 19:523–63.10. Ermak TH, Giannasca PJ, Nichols R, Myers GA, Nedrud J, Weltzin R, et al. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J Exp Med. 1998; 188:2277–88.11. Ferrero RL, Labigne A. Helicobacter pylori vaccine development in the post-genomic era: can in silico translate to in vivo. Scand J Immunol. 2001; 53:443–8.12. Ferrero RL, Thiberge JM, Kansau I, Wuscher N, Huerre M, Labigne A. The GroES homolog of Helicobacter pylori confers protective immunity against mucosal infection in mice. Proc Natl Acad Sci USA. 1995; 92:6499–503.13. Hocking D, Webb E, Radcliff F, Rothel L, Taylor S, Pinczower G, et al. Isolation of recombinant protective Helicobacter pylori antigens. Infect Immun. 1999; 67:4713–9.14. Choi SS, Chi WJ, Lee JH, Kang SS, Jeong BC, Hong SK. Overexpression of the sprD gene encoding Streptomyces griseus protease D stimulates actinorhodin production in Streptomyces lividans. J Microbiology. 2001; 39:305–13.15. Blanchard TG, Czinn SJ, Redline RW, Sigmund N, Harriman G, Nedrud JG. Antibody-independent protective mucosal immunity to gastric Helicobacter infection in mice. Cell Immunol. 1999; 191:74–80.16. Lee JH. Cloning and expression of Mammaglobin gene. Korean J Food Nutr. 2004; 17:47–52.17. Ndip RN, Malange AE, Akoachere JF, MacKay WG, Titanji VP, Weaver LT. Helicobacter pylori antigens in the faeces of asymptomatic children in the Buea and Limbe health districts of Cameroon: a pilot study. Trop Med Int Health. 2004; 9:1036–40.18. Kim PS, Lee JW, Pai SH, Kim YB, Cho JK, Lee DH, et al. Detection of Helicobacter pylori Antigen in Stool by Enzyme Immunoassay. Yonsei Med J. 2002; 43:7–13.19. Kimmel B, Bosserhoff A, Frank R, Gross R, Goebel W, Beier D. Identification of immunodominant antigens from Helicobacter pylori and evaluation of their reactivities with sera from patients with different gastroduodenal pathologies. Infect Immun. 2000; 68:915–20.20. Alm RA, Ling LS, Moir DT, King BL, Brown ED, Doig PC, et al. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999; 397:176–80.21. Sabarth N, Hurwitz R, Meyer TF, Bumann D. Multiparameter Selection of Helicobacter pylori Antigens Identifies Two Novel Antigens with High Protective Efficacy. Infect Immun. 2002; 70:6499–503.22. Asahi M, Tanaka Y, Izumi T, Ito Y, Naiki H, Kersulyte D, et al. Helicobacter pylori CagA containing ITAM-like sequences localized to lipid rafts negatively regulates VacA-induced signaling in vivo. Helicobacter. 2003; 8:1–14.23. Haas G, Karaali G, Ebermayer K, Metzger WG, Lamer S, Zimny-Arndt U, et al. Immunoproteomics of Helicobacter pylori infection and relation to gastric disease. Proteomics. 2002; 2:313–24.24. Bumann D, Aksu S, Wendland M, Janek K, Zimny-Arndt U, Sabarth N, et al. Proteome analysis of secreted proteins of the gastric pathogen Helicobacter pylori. Infect Immun. 2002; 70:3396–403.25. Cao P, McClain MS, Forsyth MH, Cover TL. Extracellular release of antigenic proteins by Helicobacter pylori. Infect Immun. 1998; 66:2984–6.26. Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, et al. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993; 90:5791–5.27. McAtee CP, Lim MY, Fung K, Velligan M, Fry K, Chow T, et al. Identification of potential diagnostic and vaccine candidates of Helicobacter pylori by two-dimensional gel electrophoresis, sequence analysis, and serum profiling. Clin Diagn Lab Immunol. 1998; 5:537–42.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Purification of the urease of helicobacter pylori and production of monoclonal antibody to the urease of helicobacter pylori
- Production of the monoclonal antibody and the genomic library of helicobacter pylori
- The value of Helicobacter pylori IgG antibody in estimating the severity of gastritis in children
- Response to Treatment of Helicobacter pylori-associated Dyspepsia: Eradication of Helicobacter pylori or Correction of Gastric or Intestinal Dysbiosis?
- Regulation of Helicobacter pylori-induced Interleukin-8 Production by Gastric Epithelial Cells in vitro