Korean Circ J.
2009 Apr;39(4):151-156. 10.4070/kcj.2009.39.4.151.
Losartan/Hydrochlorothiazide Fixed Combination Versus Amlodipine Monotherapy in Korean Patients With Mild to Moderate Hypertension
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, College of Medicine, Seoul National University, Seoul, Korea. hylee612@snu.ac.kr
- 2Division of Cardiology, Department of Internal Medicine, College of Medicine, Chungnam National University, Daejeon, Korea.
- 3Division of Cardiology, Department of Internal Medicine, College of Medicine, Pusan National University, Busan, Korea.
- 4Division of Cardiology, Department of Internal Medicine, College of Medicine, Chonnam National University, Gwangju, Korea.
- 5Division of Cardiology, Department of Internal Medicine, College of Medicine, Chungbuk National University, Cheongju, Korea.
- KMID: 2225690
- DOI: http://doi.org/10.4070/kcj.2009.39.4.151
Abstract
- BACKGROUND AND OBJECTIVES
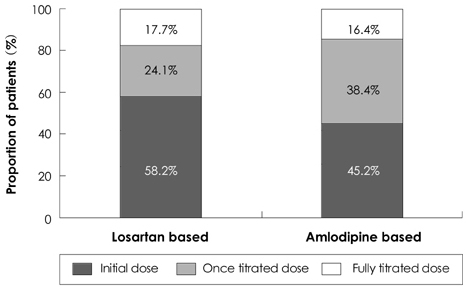
The antihypertensive efficacy and tolerability of losartan (LST) in fixed combination with hydrochlorothiazide (HCTZ) has not been compared to those of amlodipine monotherapy in Asians. This is an important comparison to draw, because Asians have been suggested to respond more favorably to calcium channel blockers and less favorably to angiotensin-converting enzyme inhibitors in comparison to Westerners. We sought to compare these two regimens in Korean patients with mild to moderate hypertension. SUBJECTS AND METHODS: 174 patients were randomized to receive LST 50 mg once daily, which could be titrated to LST/HCTZ 50/12.5 mg at 4 weeks, followed by 100/25 mg at 8 weeks; or to receive amlodipine besylate 2.5 mg once daily, which could be titrated to 5 mg at 4 weeks, followed by 10 mg at 8 weeks to achieve diastolic blood pressure <90 mmHg. RESULTS: At 12 weeks, the differences between the LST/HCTZ and amlodipine groups with regard to diastolic and systolic blood pressure were 1.2 mmHg (95% confidence interval: -1.1 to 3.4) and -0.5 mmHg (95% confidence interval: -4.3 to 3.4), respectively. The rates of achieving systolic blood pressure <140 mmHg were 66.7% in the LST/HCTZ group and 75.9% in the amlodipine group (p=0.20). The rates of drug-related adverse events were 15.6% in the LST/HCTZ group and 11.9% in the amlodipine group (p=0.49). CONCLUSION: The two regimens, with a relatively higher dose of LST/HCTZ compared to that required in Westerners, produced equivalent blood pressure reduction and were comparably well tolerated in Korean patients with mild to moderate hypertension.
Keyword
MeSH Terms
-
Amlodipine
Angiotensin-Converting Enzyme Inhibitors
Asian Continental Ancestry Group
Blood Pressure
Calcium Channel Blockers
Drug Combinations
Humans
Hydrochlorothiazide
Hypertension
Losartan
Amlodipine
Angiotensin-Converting Enzyme Inhibitors
Calcium Channel Blockers
Drug Combinations
Hydrochlorothiazide
Losartan
Figure
Reference
-
1. Dahlof B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005. 366:895–906.2. Bangalore S, Kamalakkannan G, Parkar S, Messerli FH. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med. 2007. 120:713–719.3. Waeber B, Burnier M, Brunner HR. Compliance with antihypertensive therapy. Clin Exp Hypertens. 1999. 21:973–985.4. Ruilope LM, Simpson RL, Toh J, Arcuri KE, Goldberg AI, Sweet CS. Controlled trial of losartan given concomitantly with different doses of hydrochlorothiazide in hypertensive patients. Blood Press. 1996. 5:32–40.5. Soffer BA, Wright JT Jr, Pratt JH, Wiens B, Goldberg AI, Sweet CS. Effects of losartan on a background of hydrochlorothiazide in patients with hypertension. Hypertension. 1995. 26:112–117.6. Martina B, Dieterle T, Weinbacher M, Battegay E. Effects of losartan titrated to losartan/hydrochlorothiazide and amlodipine on left ventricular mass in patients with mild-to-moderate hypertension: a double-blind randomized controlled study. Cardiology. 1999. 92:110–114.7. Wilson TW, Lacourciere Y, Barnes CC. The antihypertensive efficacy of losartan and amlodipine assessed with office and ambulatory blood pressure monitoring. CMAJ. 1998. 159:469–476.8. Phillips RA, Kloner RA, Grimm RH Jr, Weinberger M. The effects of amlodipine compared to losartan in patients with mild to moderately severe hypertension. J Clin Hypertens (Greenwich). 2003. 5:17–23.9. Dahlof B, Lindholm LH, Carney S, Pentikainen PJ, Ostergren J. Main results of the losartan versus amlodipine (LOA) study on drug tolerability and psychological general well-being. J Hypertens. 1997. 15:1327–1335.10. Jamerson K, DeQuattro V. The impact of ethnicity on response to antihypertensive therapy. Am J Med. 1996. 101:22S–32S.11. Lee HY, Kang HJ, Koo BK, et al. Clinic blood pressure responses to two amlodipine salt formulations, adipate and besylate, in adult Korean patients with mild to moderate hypertension: a multicenter, randomized, double-blind, parallel-group, 8-week comparison. Clin Ther. 2005. 27:728–739.12. Watanabe S, Okura T, Kurata M, et al. The effect of losartan and amlodipine on serum adiponectin in Japanese adults with essential hypertension. Clin Ther. 2006. 28:1677–1685.13. Yasuda G, Ando D, Hirawa N, Umemura S, Tochikubo O. Effects of losartan and amlodipine on urinary albumin excretion and ambulatory blood pressure in hypertensive type 2 diabetic patients with overt nephropathy. Diabetes Care. 2005. 28:1862–1868.14. Wu SC, Liu CP, Chiang HT, Lin SL. Prospective and randomized study of the antihypertensive effect and tolerability of three antihypertensive agents, losartan, amlodipine, and lisinopril, in hypertensive patients. Heart Vessels. 2004. 19:13–18.15. Iino Y, Hayashi M, Kawamura T, et al. Renoprotective effect of losartan in comparison to amlodipine in patients with chronic kidney disease and hypertension. Hypertens Res. 2004. 27:21–30.16. Park HC, Xu ZG, Choi S, et al. Effect of losartan and amlodipine on proteinuria and transforming growth factor-beta1 in patients with IgA nephropathy. Nephrol Dial Transplant. 2003. 18:1115–1121.17. Ishimitsu T, Minami J, Yoshii M, et al. Comparison of the effects of amlodipine and losartan on 24-hour ambulatory blood pressure in hypertensive patients. Clin Exp Hypertens. 2002. 24:41–50.18. Osterloh I. The safety of amlodipine. Am Heart J. 1989. 118:1114–1119. discussion 1119-20.19. Volpe M, Junren Z, Maxwell T, et al. Comparison of the blood pressure-lowering effects and tolerability of losartan- and amlodipine-based regimens in patients with isolated systolic hypertension. Clin Ther. 2003. 25:1469–1489.20. Leonetti G, Magnani B, Pessina AC, Rappelli A, Trimarco B, Zanchetti A. Tolerability of long-term treatment with lercanidipine versus amlodipine and lacidipine in elderly hypertensives. Am J Hypertens. 2002. 15:932–940.21. Oparil S, Barr E, Elkins M, Liss C, Vrecenak A, Edelman J. Efficacy, tolerability, and effects on quality of life of losartan, alone or with hydrochlorothiazide, versus amlodipine, alone or with hydrochlorothiazide, in patients with essential hypertension. Clin Ther. 1996. 18:608–625.22. Omvik P, Thaulow E, Herland OB, Eide I, Midha R, Turner RR. Double-blind, parallel, comparative study on quality of life during treatment with amlodipine or enalapril in mild or moderate hypertensive patients: a multicentre study. J Hypertens. 1993. 11:103–113.23. Hong SJ, Ahn TH, Baek SH, et al. Comparison of efficacy and tolerability of amlodipine orotate versus amlodipine besylate in adult patients with mild to moderate hypertension: a multicenter, randomized, double-blind, placebo-controlled, parallel-group, 8-week follow-up, noninferiority trial. Clin Ther. 2006. 28:537–551.24. Park S, Chung N, Kwon J, et al. Results of a multicenter, 8-week, parallel-group, randomized, double-blind, double-dummy, phase III clinical trial to evaluate the efficacy and tolerability of amlodipine maleate versus amlodipine besylate in Korean patients with mild to moderate hypertension. Clin Ther. 2005. 27:441–450.25. Goldberg AI, Dunlay MC, Sweet CS. Safety and tolerability of losartan potassium, an angiotensin II receptor antagonist, compared with hydrochlorothiazide, atenolol, felodipine ER, and angiotensin-converting enzyme inhibitors for the treatment of systemic hypertension. Am J Cardiol. 1995. 75:793–795.26. Gradman AH, Brady WE, Gazdick LP, Lyle P, Zeldin RK. A multicenter, randomized, double-blind, placebo-controlled, 8-week trial of the efficacy and tolerability of once-daily losartan 100 mg/hydrochlorothiazide 25 mg and losartan 50 mg/hydrochlorothiazide 12.5 mg in the treatment of moderate-to-severe essential hypertension. Clin Ther. 2002. 24:1049–1061.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Amlodipine Vs. hydrochlorothiazide: is there any disparity in its antihypertensive effect or not?
- Pharmacokinetic comparison between a fixed-dose combination of fimasartan/amlodipine/ hydrochlorothiazide 60/10/25 mg and a corresponding loose combination of fimasartan/amlodipine 60/25 mg and hydrochlorothiazide 25 mg in healthy subjects
- odipine Monotherapy in Patients with Mild to Moderate Essential Hypertension
- Chlorthalidone, not hydrochlorothiazide, is the right diuretic for comparison
- Evaluation of the effects of amlodipine on ambulatory blood pressure in hypertensive patients