Korean Circ J.
2009 Nov;39(11):477-481. 10.4070/kcj.2009.39.11.477.
Relationship Between Post-Systolic Motion During Dobutamine Stress Echocardiography and Functional Recovery of Myocardium After Successful Percutaneous Coronary Intervention
- Affiliations
-
- 1Division of Cardiology, College of Medicine, Catholic University of Daegu, Daegu, Korea. kks7379@cu.ac.kr
- KMID: 2225650
- DOI: http://doi.org/10.4070/kcj.2009.39.11.477
Abstract
- BACKGROUND AND OBJECTIVES
Doppler myocardial imaging (DMI) has been suggested as a method of quantifying inducible ischemia during dobutamine stress echocardiography (DSE). Post-systolic motion (PSM) detected by DMI is related to peri-infarct ischemia during DSE. We hypothesized that PSM during DSE would predict recovery of dysfunctional myocardium after successful percutaneous coronary intervention (PCI).
SUBJECTS AND METHODS
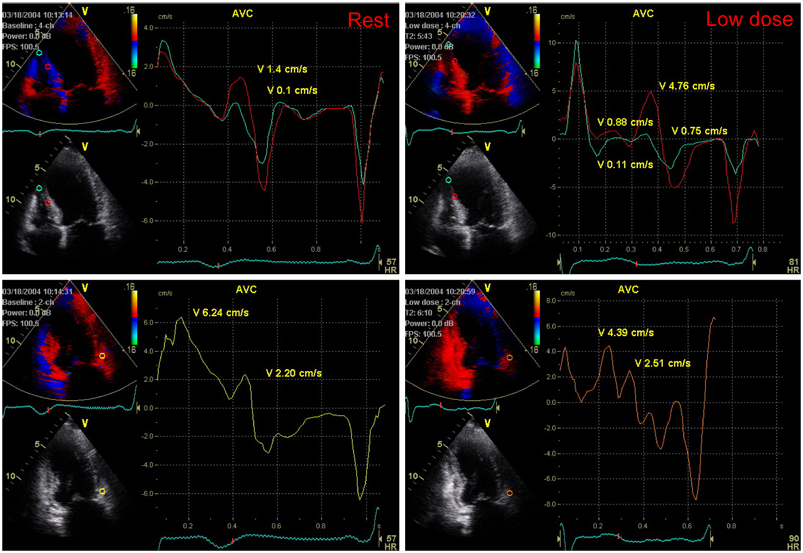
Thirty patients with dysfunctional myocardium in the left anterior descending coronary artery (LAD) territory were divided into two groups according to improvement of wall motion score index (WMSI) in the LAD territory at 6 months after successful PCI of the LAD. DMI was evaluated in the LAD territory during DSE. Fifteen patients showed improved WMSI (1.42+/-0.39) while the other 15 had unchanged WMSI (1.75+/-0.46) 1 month after PCI. Myocardial velocity was measured in the mid-septal, apico-septal, and basal anterior segments of the LAD artery territory. PSM was defined as a positive wave appearing after the curve of systolic ejection had reached the zero line.
RESULTS
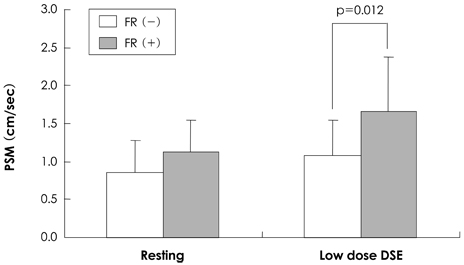
Although there was no difference between resting PSMs in both groups, PSM during DSE was significantly higher in the improved WMSI group than in the WMSI group where it was unchanged.
CONCLUSION
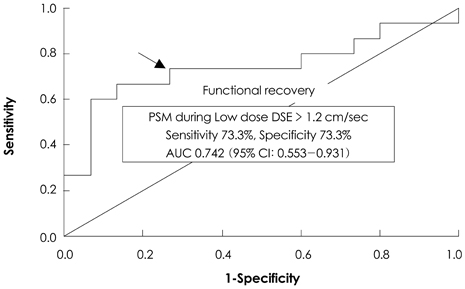
PSM during DSE predicts recovery of dysfunctional myocardium after successful PCI.
MeSH Terms
Figure
Reference
-
1. van den Berg EK Jr, Popma JJ, Dehmer GJ, et al. Reversible segmental left ventricular dysfunction after coronary angioplasty. Circulation. 1990. 81:1210–1216.2. Rahimtoola SH. The hibernating myocardium. Am Heart J. 1989. 117:211–221.3. Jeong MH, Cha KS, Seo JP, et al. Hibernating myocardium in chronic coronary artery disease. Korean Circ J. 1997. 27:206–212.4. Nishio M, Tanouchi J, Tanaka K, et al. Dobutamine stress echocardiography at 7.5 µg/kg/min using color tissue Doppler imaging M-mode safely predicts reversible dysfunction early after reperfusion in patients with acute myocardial infarction. Am J Cardiol. 1999. 83:340–344.5. Kim KS. The usefulness of dobutamine stress echocardiography for evaluation of viable myocardium in hibernating myocardium. Korean Circ J. 1998. 28:1233–1236.6. Lee SH, Park H, Son MS, et al. Evaluation of Doppler echocardiographic patterns of left ventricular filling in the patients with recent acute myocardial infarction. Korean Circ J. 1993. 23:223–229.7. Brown MA, Norris RM, Takayama M, White HD. Post-systolic shortening: a marker of potential for early recovery of acutely ischemic myocardium in the dog. Cardiovasc Res. 1987. 21:703–716.8. Takayama M, Norris RM, Brown MA, Armiger LC, Rivers JT, White HD. Postsystolic shortening of acutely ischemic canine myocardium predicts early and late recovery of function following coronary artery reperfusion. Circulation. 1988. 78:994–1007.9. Kass DA, Midei M, Brinker J, Maughan WL. Influence of coronary occlusion during PTCA on end-systolic and end-diastolic pressure-volume relations in humans. Circulation. 1990. 81:447–460.10. Hosokawa H, Sheenhan FH, Suzuki T. Measurement of postsystolic shortening to assess viability and predict recovery of left ventricular function after acute myocardial infarction. J Am Coll Cardiol. 2000. 35:1842–1849.11. Cain P, Baglin T, Spicer D, Short L, Marwick TH. Application of tissue Doppler to interpretation of dobutamine echocardiography and comparison with quantitative coronary angiography. Am J Cardiol. 2001. 87:525–531.12. Celutkiene J, Sutherland GR, Laucevicius A, Zakarkaite D, Rudys A, Grabauskiene V. Is post-systolic motion the optimal ultrasound parameter to detect induced ischaemia during dobutamine stress echocardiography? Eur Heart J. 2004. 25:932–942.13. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005. 18:1440–1463.14. Song JM, Kim JH, Kim YH, et al. Temporal changes and histologic relation of postsystolic thickening in an animal model of acute ischemia and reperfusion. J Am Soc Echocardiogr. 2003. 16:409–414.15. Citro R, Galderisi M. Myocardial postsystolic motion in ischemic and not ischemic myocardium: the clinical value of tissue Doppler. Echocardiography. 2005. 22:525–532.16. Hosokawa H, Sheehan FH, Suzuki T. Measurement of postsystolic shortening to assess viability and predict recovery of left ventricular function after acute myocardial infarction. J Am Coll Cardiol. 2000. 35:1842–1849.17. Song JK, Song JM, Kang DH, Haluska B, Marwick TH. Postsystolic thickening detected by Doppler myocardial imaging: a marker of viability or ischemia in patients with myocardial infarction. Clin Cardiol. 2004. 27:29–32.18. Wong CK, Freedman SB, Bautovich G, Hutton BF. Correlation between post-ejection shortening and improvement in regional wall motion after revascularization in patients with coronary artery disease. Int J Cardiol. 1996. 54:61–67.19. Barletta G, Del Bene R, Lo Sapio P, Gallini C, Fantini F. Postejection thickening as a marker of viable myocardium: an echocardiographic study in patients with chronic coronary artery disease. Basic Res Cardiol. 1998. 93:313–324.20. Lim P, Pasquet A, Gerber B, et al. Is postsystolic shortening a marker of viability in chronic left ventricular ischemic dysfunction?: comparison with late enhancement contrast magnetic resonance imaging. J Am Soc Echocardiogr. 2008. 21:452–457.21. Akutsu Y, Shinozuka A, Kodama Y, et al. Usefulness of simultaneous evaluations of contractile reserve, perfusion, and metabolism during dobutamine stress for predicting wall motion reversibility (myocardial stunning) after successful PTCA. Jpn Heart J. 2004. 45:195–204.22. Rambaldi R, Bax JJ, Rizzello V, et al. Post-systolic shortening during dobutamine stress echocardiography predicts cardiac survival in patients with severe left ventricular dysfunction. Coron Artery Dis. 2005. 16:141–145.23. Pigott JD, Kouchoukos NT, Oberman A, Cutter GR. Late results of surgical and medical therapy for patients with coronary artery disease and depressed left ventricular function. J Am Coll Cardiol. 1985. 5:1036–1045.24. Boden WE, Brooks WW, Conrad CH, Bing OH, Hood WB Jr. Incomplete, delayed functional recovery late after reperfusion following acute myocardial infarction: "maimed myocardium". Am Heart J. 1995. 130:922–932.25. Rambaldi R, Poldermans D, Fioretti PM, et al. Usefulness of pulse-wave Doppler tissue sampling and dobutamine stress echocardiography for the diagnosis of right coronary artery narrowing. Am J Cardiol. 1998. 81:1411–1415.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dobutamine Echocardiography in Chronic Coronary Artery Disease with Left Ventricular Dysfunction
- The Usefulness of Dobutamine Stress Echocardiography for Evaluation of Viable Myocardium in Hibernating Myocardium
- Identification of ischemic myocardium with simultaneous dobutamine stress echocardiography and 99mTc-MIBI SPECT in patients with suspected coronary artery disease
- Identification of Ischemic Myocardium with Simultaneous Dobutamine Stress Echocardiography and 99mTc-MIBI SPECT in Patients with Suspected Coronary Artery Disease
- Prediction of Improvement of Hibernating Myocardium after Coronary Artery Bypass Grafting: The role of dobutamine stress echocardiography