Korean Circ J.
2013 Mar;43(3):161-167. 10.4070/kcj.2013.43.3.161.
Long-Term Predictors of Clinical Events after Off-Label Use of Drug-Eluting Stent beyond 1 Year
- Affiliations
-
- 1Department of Cardiovascular Medicine, Regional Cardiovascular Center, Wonkwang University Hospital, Iksan, Korea. ards7210@yahoo.co.kr
- KMID: 2224959
- DOI: http://doi.org/10.4070/kcj.2013.43.3.161
Abstract
- BACKGROUND AND OBJECTIVES
We evaluated the long-term outcomes and predictors of clinical events after off-label use of drug-eluting stents (DES) beyond 1 year after procedure.
SUBJECTS AND METHODS
A total of 518 patients who underwent DES implantation for off-label indications and did not have any major adverse cardiac events (MACE) during the first year were analyzed. The occurrence of MACE, including cardiac death, myocardial infarction (MI), stent thrombosis and target vessel revascularization, were evaluated for a median 1179 days (interquartile range 769-1541) after the first year.
RESULTS
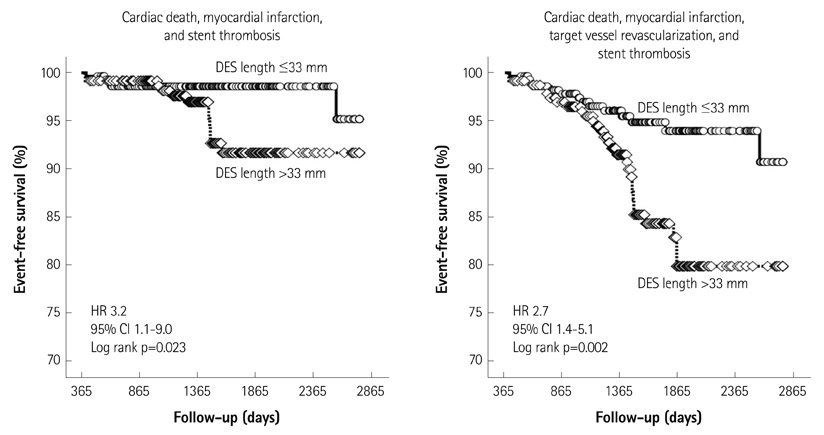
Major adverse cardiac events occurred in 43 patients (8.3%) including 8 cases (1.5%) of cardiac death, 9 cases (1.7%) of MI, 24 cases (4.6%) of target vessel revascularization, and 11 cases (2.1%) of stent thrombosis. Patients with MACE had a higher serum creatinine level, higher incidence of in-stent restenosis lesion, more overlapping stents, a greater number of stents, and longer stents than did patients without MACE. Multivariate analysis revealed that serum creatinine level >1.5 mg/dL {hazard ratio (HR) 2.3, p=0.019}, stent length >33 mm (HR 2.4, p=0.035), and in-stent restenosis lesions (HR 2.4, p=0.040) were independent risk factors for MACE. Patients with DES length >33 mm had a higher incidence of MACE than those with DES length < or =33 mm (HR 2.7, log rank p=0.002).
CONCLUSION
The risk of stent thrombosis and target vessel revascularization persisted in patients undergoing off-label DES implantation beyond 1-year follow-up. A total DES length >33 mm was a significant procedural predictor associated with the incidence of MACE.
MeSH Terms
Figure
Reference
-
1. Babapulle MN, Joseph L, Bélisle P, Brophy JM, Eisenberg MJ. A hierarchical Bayesian meta-analysis of randomised clinical trials of drug-eluting stents. Lancet. 2004. 364:583–591.2. Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003. 349:1315–1323.3. Stone GW, Ellis SG, Cox DA, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004. 350:221–231.4. Beohar N, Meyers SN, Erdogan A, et al. Off-label use of drug-eluting versus bare metal stents: a lesion-specific systematic review of long-term outcomes. J Interv Cardiol. 2010. 23:528–545.5. Serruys PW, Onuma Y, Garg S, et al. 5-year clinical outcomes of the ARTS II (Arterial Revascularization Therapies Study II) of the sirolimus-eluting stent in the treatment of patients with multivessel de novo coronary artery lesions. J Am Coll Cardiol. 2010. 55:1093–1101.6. Ellis SG, Stone GW, Cox DA, et al. Long-term safety and efficacy with paclitaxel-eluting stents: 5-year final results of the TAXUS IV clinical trial (TAXUS IV-SR: Treatment of De Novo Coronary Disease Using a Single Paclitaxel-Eluting Stent). JACC Cardiovasc Interv. 2009. 2:1248–1259.7. Caixeta A, Leon MB, Lansky AJ, et al. 5-year clinical outcomes after sirolimus-eluting stent implantation insights from a patient-level pooled analysis of 4 randomized trials comparing sirolimus-eluting stents with bare-metal stents. J Am Coll Cardiol. 2009. 54:894–902.8. Win HK, Caldera AE, Maresh K, et al. Clinical outcomes and stent thrombosis following off-label use of drug-eluting stents. JAMA. 2007. 297:2001–2009.9. Planer D, Beyar R, Almagor Y, et al. Long-term (>3 Years) outcome and predictors of clinical events after insertion of sirolimus-eluting stent in one or more native coronary arteries (from the Israeli arm of the e-Cypher registry). Am J Cardiol. 2008. 101:953–959.10. Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS, Mehran R. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 2010. 56:1897–1907.11. Lee CW, Park SJ. Predictive factors for restenosis after drug-eluting stent implantation. Korean Circ J. 2007. 37:97–102.12. Park K, Park KW, Rha SW, et al. Comparison of 5-year clinical outcomes between sirolimus-versus paclitaxel-eluting stent: Korean multicenter network analysis of 9000-patient cohort. Circ Cardiovasc Interv. 2012. 5:174–184.13. Park KW, Kim CH, Lee HY, et al. Does "late catch-up" exist in drug-eluting stents: insights from a serial quantitative coronary angiography analysis of sirolimus versus paclitaxel-eluting stents. Am Heart J. 2010. 159:446–453.14. Ko YG, Kim JS, Choi D, et al. Five-year outcomes of sirolimus-eluting versus paclitaxel-eluting stents: a propensity matched study: clinical evidence of late catch-up? Int J Cardiol. 2011. 152:302–306.15. Brodie BR, Stuckey T, Downey W, et al. Outcomes and complications with off-label use of drug-eluting stents: results from the STENT (Strategic Transcatheter Evaluation of New Therapies) group. JACC Cardiovasc Interv. 2008. 1:405–414.16. Weisz G, Leon MB, Holmes DR Jr, et al. Five-year follow-up after sirolimus-eluting stent implantation results of the SIRIUS (Sirolimus-Eluting Stent in De-Novo Native Coronary Lesions) Trial. J Am Coll Cardiol. 2009. 53:1488–1497.17. Kandzari DE, Mauri L, Popma JJ, et al. Late-term clinical outcomes with zotarolimus- and sirolimus-eluting stents. 5-year follow-up of the ENDEAVOR III (A Randomized Controlled Trial of the Medtronic Endeavor Drug [ABT-578] Eluting Coronary Stent System Versus the Cypher Sirolimus-Eluting Coronary Stent System in De Novo Native Coronary Artery Lesions). JACC Cardiovasc Interv. 2011. 4:543–550.18. Nikol S, Huehns TY, Höfling B. Molecular biology and post-angioplasty restenosis. Atherosclerosis. 1996. 123:17–31.19. Serruys PW, Daemen J, Morice MC, et al. Three-year follow-up of the ARTS-II# - sirolimus-eluting stents for the treatment of patients with multivessel coronary artery disease. EuroIntervention. 2008. 3:450–459.20. Suh J, Park DW, Lee JY, et al. The relationship and threshold of stent length with regard to risk of stent thrombosis after drug-eluting stent implantation. JACC Cardiovasc Interv. 2010. 3:383–389.21. Kim YH, Park SW, Lee SW, et al. Sirolimus-eluting stent versus paclitaxel-eluting stent for patients with long coronary artery disease. Circulation. 2006. 114:2148–2153.22. Dawkins KD, Grube E, Guagliumi G, et al. Clinical efficacy of polymer-based paclitaxel-eluting stents in the treatment of complex, long coronary artery lesions from a multicenter, randomized trial: support for the use of drug-eluting stents in contemporary clinical practice. Circulation. 2005. 112:3306–3313.23. Colombo A, De Gregorio J, Moussa I, et al. Intravascular ultrasound-guided percutaneous transluminal coronary angioplasty with provisional spot stenting for treatment of long coronary lesions. J Am Coll Cardiol. 2001. 38:1427–1433.24. Katritsis DG, Korovesis S, Tzanalaridou E, Giazitzoglou E, Voridis E, Meier B. Comparison of long versus short ("spot") drug-eluting stenting for long coronary stenoses. Am J Cardiol. 2009. 104:786–790.25. Katritsis DG, Korovesis S, Tzanalaridou E, Giazitzoglou E, Zografos T, Meier B. Spot drug-eluting stenting for long coronary stenoses: long-term results of a randomized clinical study. J Interv Cardiol. 2011. 24:437–441.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Drug-Eluting Stent Strut Fracture as a Cause of Restenosis
- Five-year clinical outcomes of drug-eluting stents according to on-label and off-label use
- Dark Side of Drug-eluting Stent in Contemporary Percutaneous Coronary Intervention
- Drug-Eluting Stent: Present and Future
- A Case of Stent Strut Fracture of a Paclitaxel-Eluting Stent at the Time of Stent Implantation in a Complex Coronary Lesion