J Rheum Dis.
2013 Oct;20(5):303-309. 10.4078/jrd.2013.20.5.303.
Rituximab Treatment for the Patients with Refractory Inflammatory Myopathy
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. elee@snu.ac.kr
- KMID: 2223006
- DOI: http://doi.org/10.4078/jrd.2013.20.5.303
Abstract
OBJECTIVE
To assess the efficacy and safety of rituximab (RTX) on disease activity and muscle strength in patients with inflammatory myopathies refractory to conventional therapy.
METHODS
Four inflammatory myopathy patients who had been refractory to glucocorticoids, one or more immunosuppressive therapies and intravenous immunoglobulin were treated on an open-label basis. Each patient received two 500 mg doses of RTX 2 weeks apart in one cycle. In one patient who did not respond after the first cycle of RTX, the infusion schedule was modified by the physician. We measured muscle enzyme including CPK, LDH and assessed muscle strength individually to evaluate RTX response. Additionally anti-CD19 antibody was measured.
RESULTS
Three patients responded to the first cycle of RTX treatment with improvements in muscle enzyme and muscle strength, and then maintained physical function over the duration of several infusion cycles. In one patient, muscle enzyme did not decrease after the first cycle of RTX, and a high dose glucocorticoid was given. After modifying the treatment schedule with monthly RTX infusion, his muscle enzyme level and muscle strength improved. Anti-CD19 antibody decreased after RTX generally, but responses were variable. Herpes zoster infection occurred in two patients.
CONCLUSION
Rituximab may be a therapeutic choice in refractory inflammatory myopathy. However a further trial is needed to confirm the efficacy and prove the safety.
Keyword
MeSH Terms
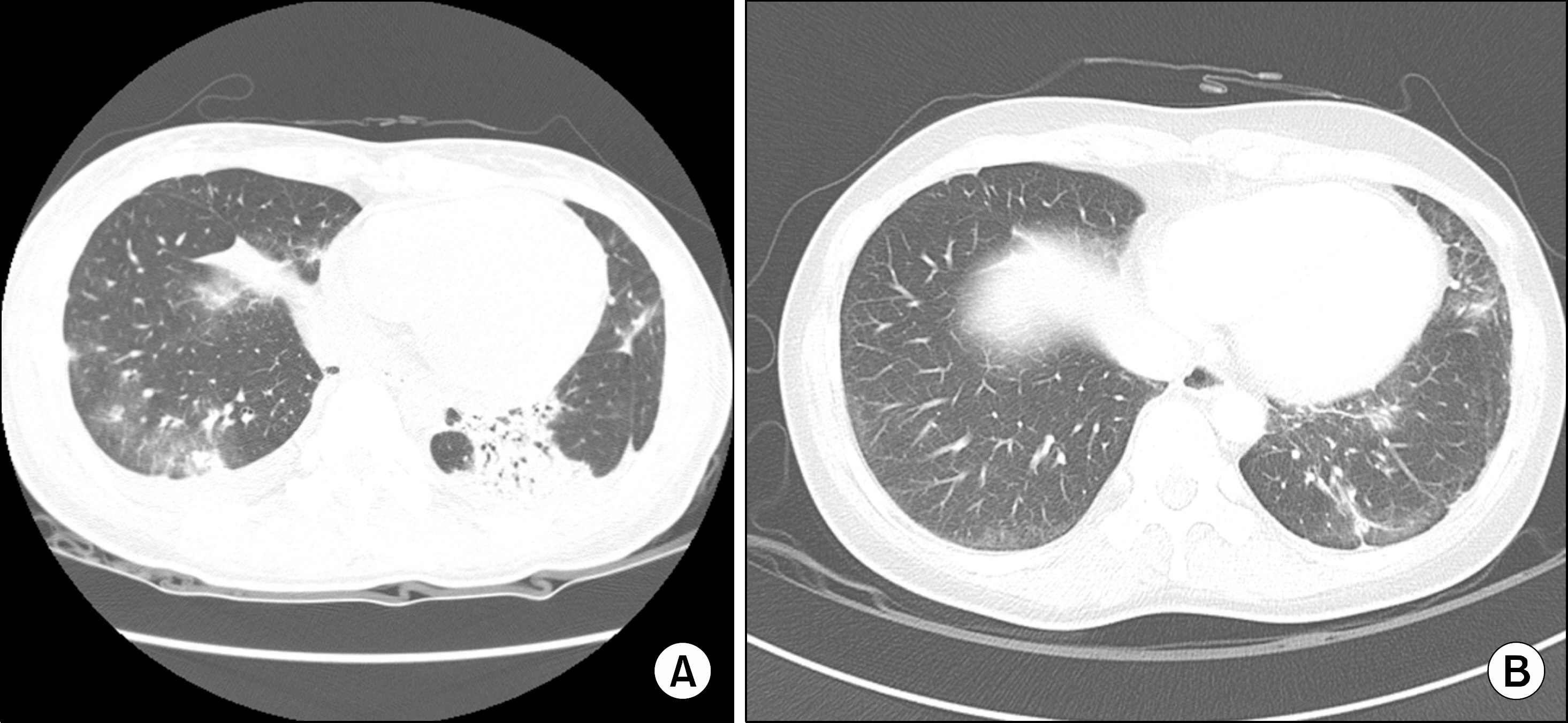
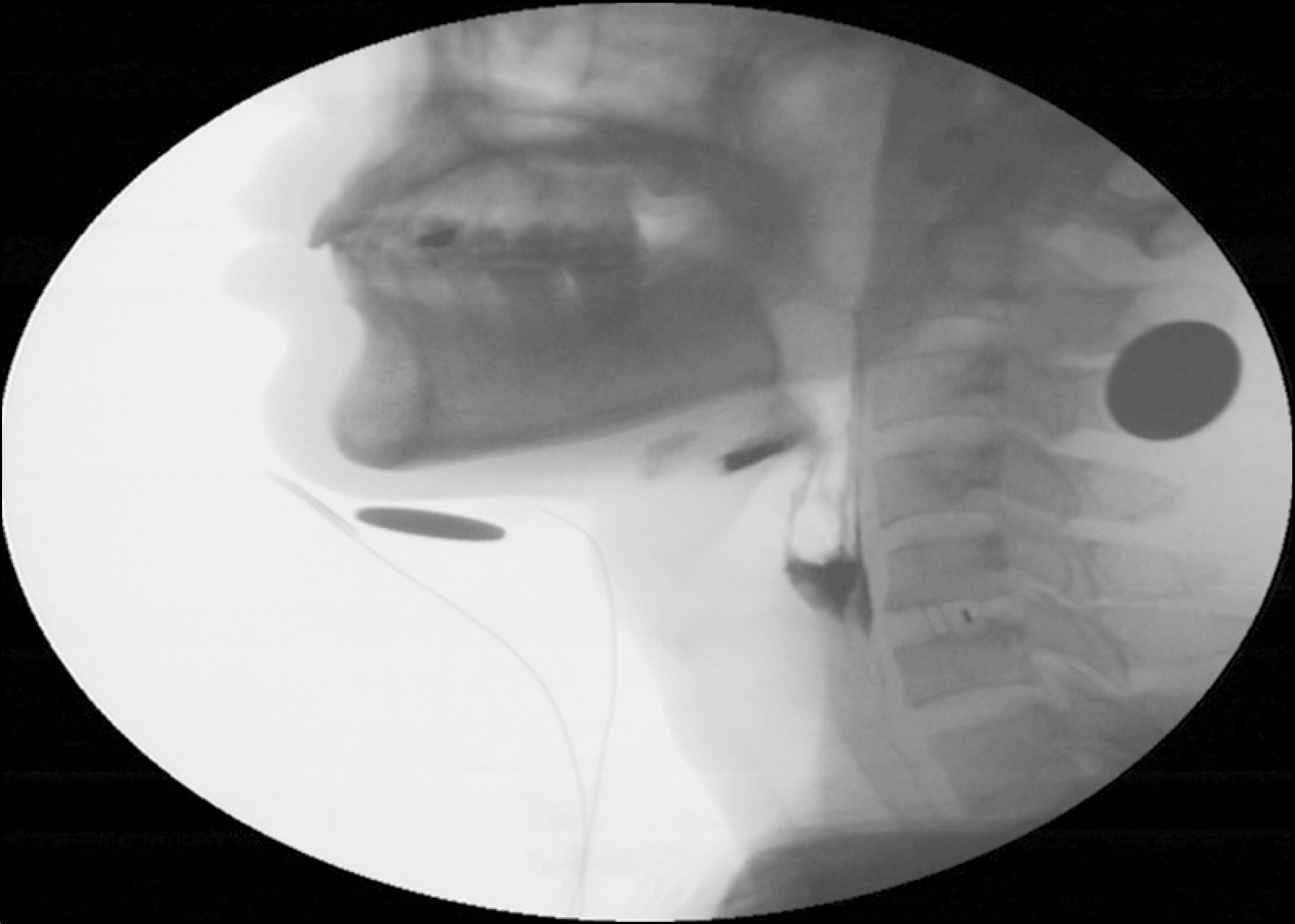
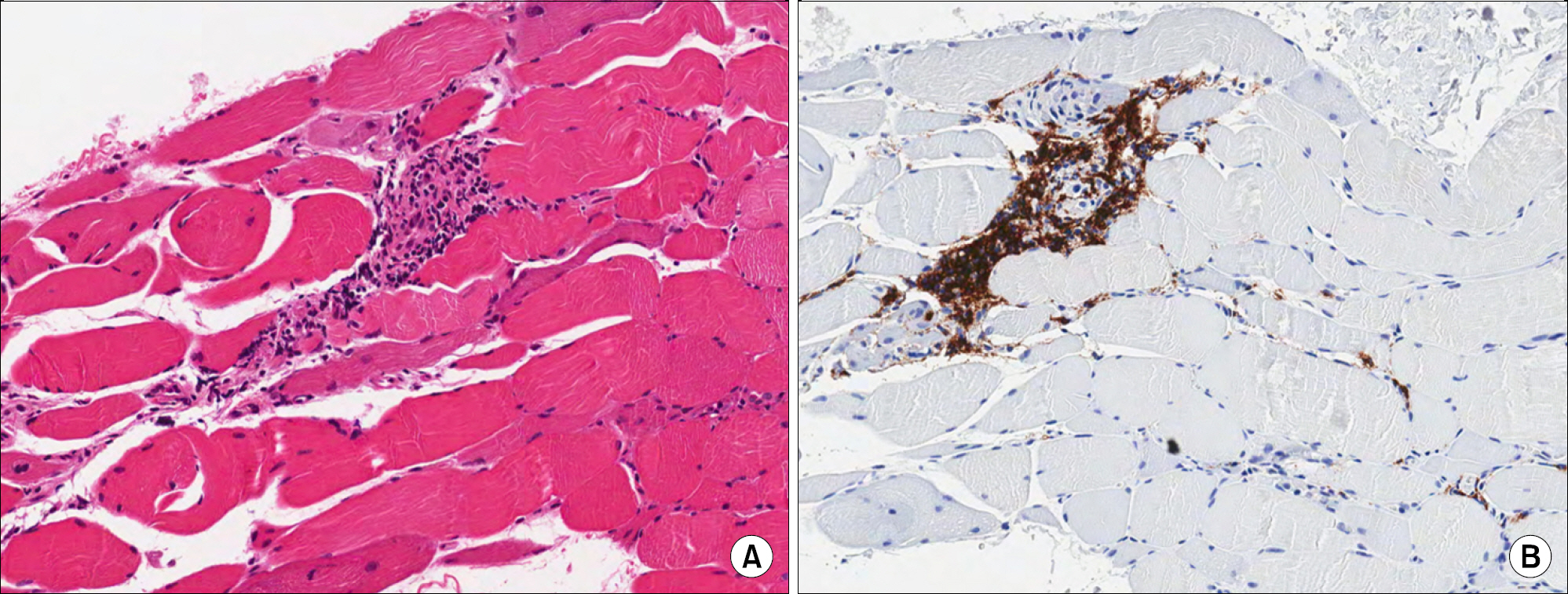
Figure
Cited by 2 articles
-
Efficacy and Safety of Rituximab in Korean Patients with Refractory Inflammatory Myopathies
Ga Young Ahn, Chang-Hee Suh, Yong-Gil Kim, Yong-Beom Park, Seung Cheol Shim, Sang-Heon Lee, Shin-Seok Lee, Sang-Cheol Bae, Dae Hyun Yoo
J Korean Med Sci. 2020;35(38):e335. doi: 10.3346/jkms.2020.35.e335.Rituximab in Patients with Inflammatory Myopathies
Seong Wook Kang
J Rheum Dis. 2013;20(6):345-347. doi: 10.4078/jrd.2013.20.6.345.
Reference
-
References
1. Ernste FC, Reed AM, Idiopathic inflammatory myopathies: current trends in pathogenesis, clinical features, and up-to-date treatment recommendations. Mayo Clin Proc. 2013; 88:83–105.2. Mahler EA, Blom M, Voermans NC, van Engelen BG, van Riel PL, Vonk MC. Rituximab treatment in patients with refractory inflammatory myopathies. Rheumatology (Oxford). 2011; 50:2206–13.
Article3. Pipitone N, Salvarani C. Established and new treatments of the idiopathic inflammatory myopathies: dermatomyositis and polymyositis. Clin Exp Rheumatol. 2007; 25:896–906.4. Cooper MA, Willingham DL, Brown DE, French AR, Shih FF, White AJ. Rituximab for the treatment of juvenile dermatomyositis: a report of four pediatric patients. Arthritis Rheum. 2007; 56:3107–11.
Article5. Chiappetta N, Steier J, Gruber B. Rituximab in the treatment of refractory dermatomyositis. J Clin Rheumatol. 2005; 11:264–6.
Article6. Rios Fernández R, Callejas Rubio JL, Sánchez Cano D, Sáez Moreno JA, Ortego Centeno N. Rituximab in the treatment of dermatomyositis and other inflammatory myopathies. A report of 4 cases and review of the literature. Clin Exp Rheumatol. 2009; 27:1009–16.7. Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, et al. Depletion of B cells in vivo by a chi- meric mouse human monoclonal antibody to CD20. Blood. 1994; 83:435–45.8. Bader-Meunier B, Decaluwe H, Barnerias C, Gherardi R, Quartier P, Faye A, et al. Club Rhumatismes et Inflammation. Safety and efficacy of rituximab in severe juvenile dermatomyositis: results from 9 patients from the French Autoimmunity and Rituximab registry. J Rheumatol. 2011; 38:1436–40.9. Levine TD. Rituximab in the treatment of dermatomyositis: an open-label pilot study. Arthritis Rheum. 2005; 52:601–7.
Article10. Valiyil R, Casciola-Rosen L, Hong G, Mammen A, Christopher-Stine L. Rituximab therapy for myopathy associated with anti-signal recognition particle antibodies: a case series. Arthritis Care Res (Hoboken). 2010; 62:1328–34.
Article11. Oddis CV, Reed AM, Aggarwal R, Rider LG, Ascher-man DP, Levesque MC, et al. RIM Study Group. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013; 65:314–24.12. Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975; 292:403–7.13. Rosman Z, Shoenfeld Y, Zandman-Goddard G. Biologic therapy for autoimmune diseases: an update. BMC Med. 2013; 11:88.
Article14. Majmudar S, Hall HA, Zimmermann B. Treatment of adult inflammatory myositis with rituximab: an emerging therapy for refractory patients. J Clin Rheumatol. 2009; 15:338–40.15. Chiu YE, Co DO. Juvenile dermatomyositis: immunopa-thogenesis, role of myositis-specific autoantibodies, and review of rituximab use. Pediatr Dermatol. 2011; 28:357–67.
Article16. Rider LG, Miller FW. Deciphering the clinical presentations, pathogenesis, and treatment of the idiopathic inflammatory myopathies. JAMA. 2011; 305:183–90.
Article17. Sultan SM, Ng KP, Edwards JC, Isenberg DA, Cambridge G. Clinical outcome following B cell depletion therapy in eight patients with refractory idiopathic inflammatory myopathy. Clin Exp Rheumatol. 2008; 26:887–93.18. Whelan BR, Isenberg DA. Poor response of anti-SRP-pos-itive idiopathic immune myositis to B-cell depletion. Rheumatology (Oxford). 2009; 48:594–5.
Article19. Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004; 350:2572–81.
Article20. Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, et al. REFLEX Trial Group. Rituximab for rheumatoid arthritis refractory to antitumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006; 54:2793–806.21. Emery P, Fleischmann R, Filipowicz-Sosnowska A, Schechtman J, Szczepanski L, Kavanaugh A, et al. DANCER Study Group. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum. 2006; 54:1390–400.22. Hainsworth JD. Safety of rituximab in the treatment of B cell malignancies: implications for rheumatoid arthritis. Arthritis Res Ther. 2003; 5(Suppl 4):S12–6.23. Chambers SA, Isenberg D. Anti-B cell therapy (rituximab) in the treatment of autoimmune diseases. Lupus. 2005; 14:210–4.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Remission after Rituximab Therapy in Refractory Myasthenia Gravis
- Myasthenia Gravis Occurring Simultaneously With Inflammatory Myopathy in Sjogren's Syndrome
- Rituximab Treatment for Neuroimmunologic Disorders
- Rituximab Treatment for the Patient with Refractory Lupus Nephritis
- Primary acquired chronic pure red cell aplasia refractory to standard treatments: remission with rituximab