Dement Neurocogn Disord.
2013 Mar;12(1):21-28. 10.12779/dnd.2013.12.1.21.
A Semi-Automated Method for Measuring White Matter Hyperintensity Volume
- Affiliations
-
- 1Department of Neurology, The Catholic University of Korea, Seoul, Korea. neuroman@catholic.ac.kr
- 2Department of Neurology, Konyang University College of Medicine, Daejeon, Korea.
- 3Hyoja Geriatric Hospital, Yongin, Korea.
- KMID: 2222357
- DOI: http://doi.org/10.12779/dnd.2013.12.1.21
Abstract
- BACKGROUND
White matter hyperintensities (WMHs) on magnetic resonance imaging (MRI) have been considered as a reliable biomarker of small vessel damages. To evaluate the severity of WMHs, it is vital to develop reliable methods to measure the volume of WMHs. We applied open source software to measure WMH volume in the semi-automated way, and tested the reliability and validity by comparing with the commonly used qualitative rating scale.
METHODS
Twenty five subjects with variable WMHs were recruited. ANALYZE 10.0 was used for the image processing and volumetric measurement of WMHs. The inhomogeneity and artifacts of signal were corrected with Insight Segmentation and Registration Toolkit in ANALYZE. For the gold standard of the WMH volumetric measurement, threshold method was applied with consensus of manual editing on each slice of the MRI images by two raters. Histogram of the all slices of the Fluid Attenuated Inversion Recovery (FLAIR) MRI was generated to calculate the optimal voxel intensity of threshold, and the lowest voxel threshold was decided as the mean+1.4 SD. The volumes of WMHs were generated by multiplying the area and the thickness of each slice. Inter- and intrarater reliability of the semi-automated volumetric and Scheltens'methods, and the association between the individual methods were analyzed.
RESULTS
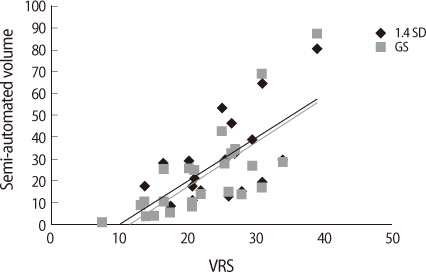
The semi-automated WMH volume at the threshold of 1.4 SD as well as the gold standard volume was well correlated with the Scheltens' visual scale (r=0.75, p<0.001). The semi-automated volumetry showed the excellent intra-rater (ICC=0.9929; 95% CI, 0.9840-0.9968) and inter-rater reliability (ICC=0.9830; 95% CI, 0.9620-0.9925), superior to the Scheltens' visual rating scale.
CONCLUSIONS
The semi-automated volume measurement of the WMHs with Analyze was a valid and a reliable method to quantify subcortical white matter damages of various etiologies.
MeSH Terms
Figure
Reference
-
1. de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001. 70:9–14.
Article2. Vermeer SE, van Dijk EJ, Koudstaal PJ, Oudkerk M, Hofman A, Clarke R, et al. Homocysteine, silent brain infarcts, and white matter lesions: The Rotterdam Scan Study. Ann Neurol. 2002. 51:285–289.
Article3. Basile AM, Pantoni L, Pracucci G, Asplund K, Chabriat H, Erkinjuntti T, et al. Age, hypertension, and lacunar stroke are the major determinants of the severity of age-related white matter changes. The LADIS (Leukoaraiosis and Disability in the Elderly) Study. Cerebrovasc Dis. 2006. 21:315–322.
Article4. de Leeuw FE, de Groot JC, van Gijn J. Cerebral white matter lesions in the elderly: vascular risk factors and cognitive consequences. Ned Tijdschr Geneeskd. 2001. 145:2067–2071.
Article5. Markus HS, Hunt B, Palmer K, Enzinger C, Schmidt H, Schmidt R. Markers of endothelial and hemostatic activation and progression of cerebral white matter hyperintensities: longitudinal results of the Austrian Stroke Prevention Study. Stroke. 2005. 36:1410–1414.
Article6. Turner ST, Jack CR, Fornage M, Mosley TH, Boerwinkle E, de Andrade M. Heritability of leukoaraiosis in hypertensive sibships. Hypertension. 2004. 43:483–487.
Article7. DeStefano AL, Atwood LD, Massaro JM, Heard-Costa N, Beiser A, Au R, et al. Genome-wide scan for white matter hyperintensity: the Framingham Heart Study. Stroke. 2006. 37:77–81.
Article8. Schmidt H, Fazekas F, Kostner GM, van Duijn CM, Schmidt R. Angiotensinogen gene promoter haplotype and microangiopathy-related cerebral damage: results of the Austrian Stroke Prevention Study. Stroke. 2001. 32:405–412.
Article9. Roman GC. Age-associated white matter lesions and dementia: are these lesions causal or casual? Arch Neurol. 2004. 61:1503–1504.
Article10. Sachdev P, Brodaty H, Rose N, Haindl W. Regional cerebral blood flow in late-onset schizophrenia: a SPECT study using 99mTc-HMPAO. Schizophr Res. 1997. 27:105–117.
Article11. Kumar A, Bilker W, Jin Z, Udupa J. Atrophy and high intensity lesions: complementary neurobiological mechanisms in late-life major depression. Neuropsychopharmacology. 2000. 22:264–274.
Article12. Schmidt R, Fazekas F, Kapeller P, Schmidt H, Hartung HP. MRI white matter hyperintensities: three-year follow-up of the Austrian Stroke Prevention Study. Neurology. 1999. 53:132–139.
Article13. Veldink JH, Scheltens P, Jonker C, Launer LJ. Progression of cerebral white matter hyperintensities on MRI is related to diastolic blood pressure. Neurology. 1998. 51:319–320.
Article14. Wahlund LO, Almkvist O, Basun H, Julin P. MRI in successful aging, a 5-year follow-up study from the eighth to ninth decade of life. Magn Reson Imaging. 1996. 14:601–608.
Article15. Prins ND, van Straaten EC, van Dijk EJ, Simoni M, van Schijndel RA, Vrooman HA, et al. Measuring progression of cerebral white matter lesions on MRI: visual rating and volumetrics. Neurology. 2004. 62:1533–1539.
Article16. Fazekas F, Barkhof F, Wahlund LO, Pantoni L, Erkinjuntti T, Scheltens P, et al. CT and MRI rating of white matter lesions. Cerebrovasc Dis. 2002. 13:31–36.
Article17. Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjögren M, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001. 32:1318–1322.
Article18. Kapeller P, Barber R, Vermeulen RJ, Adèr H, Scheltens P, Freidl W, et al. Visual rating of age-related white matter changes on magnetic resonance imaging: scale comparison, interrater agreement, and correlations with quantitative measurements. Stroke. 2003. 34:441–445.
Article19. van Straaten EC, Fazekas F, Rostrup E, Scheltens P, Schmidt R, Pantoni L, et al. Impact of white matter hyperintensities scoring method on correlations with clinical data: the LADIS study. Stroke. 2006. 37:836–840.
Article20. Payne ME, Fetzer DL, MacFall JR, Provenzale JM, Byrum CE, Krishnan KR. Development of a semi-automated method for quantification of MRI gray and white matter lesions in geriatric subjects. Psychiatry Res. 2002. 115:63–77.
Article21. Scheltens P, Barkhof F, Leys D, Pruvo JP, Nauta JJ, Vermersch P, et al. A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J Neurol Sci. 1993. 114:7–12.
Article22. Park HK, Na DL, Han SH, Kim JY, Cheong HK, Kim SY, et al. Clinical characteristics of a nationwide hospital-based registry of mild-to-moderate Alzheimer's disease patients in Korea: a CREDOS (Clinical Research Center for Dementia of South Korea) study. J Korean Med Sci. 2011. 26:1219–1226.
Article23. Hirono N, Kitagaki H, Kazui H, Hashimoto M, Mori E. Impact of white matter changes on clinical manifestation of Alzheimer's disease: A quantitative study. Stroke. 2000. 31:2182–2188.
Article24. Filippi M, Horsfield MA, Bressi S, Martinelli V, Baratti C, Reganati P, et al. Intra- and inter-observer agreement of brain MRI lesion volume measurements in multiple sclerosis. A comparison of techniques. Brain. 1995. 118:1593–1600.
Article25. van Walderveen MA, Barkhof F, Hommes OR, Polman CH, Tobi H, Frequin ST, et al. Correlating MRI and clinical disease activity in multiple sclerosis: relevance of hypointense lesions on short-TR/short-TE (T1-weighted) spin-echo images. Neurology. 1995. 45:1684–1690.26. Mitchell JR, Karlik SJ, Lee DH, Eliasziw M, Rice GP, Fenster A. The variability of manual and computer assisted quantification of multiple sclerosis lesion volumes. Medical physics. 1996. 23:85–97.27. Grimaud J, Lai M, Thorpe J, Adeleine P, Wang L, Barker GJ, et al. Quantification of MRI lesion load in multiple sclerosis: a comparison of three computer-assisted techniques. Magn Reson Imaging. 1996. 14:495–505.
Article28. Molyneux PD, Tofts PS, Fletcher A, Gunn B, Robinson P, Gallagher H, et al. Precision and reliability for measurement of change in MRI lesion volume in multiple sclerosis: a comparison of two computer assisted techniques. J Neurol Neurosurg Psychiatry. 1998. 65:42–47.
Article29. Coffey CE, Figiel GS, Djang WT, Cress M, Saunders WB, Weiner RD. Leukoencephalopathy in elderly depressed patients referred for ECT. Biol Psychiatry. 1988. 24:143–161.
Article30. Davis PC, Gray L, Albert M, Wilkinson W, Hughes J, Heyman A, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part III. Reliability of a standardized MRI evaluation of Alzheimer's disease. Neurology. 1992. 42:1676–1680.
Article31. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987. 149:351–356.
Article32. Boyko OB, Alston SR, Fuller GN, Hulette CM, Johnson GA, Burger PC. Utility of postmortem magnetic resonance imaging in clinical neuropathology. Arch Pathol Lab Med. 1994. 118:219–225.
Article33. Chen J, Reutens DC. Inhomogeneity correction for brain magnetic resonance images by rank leveling. J Comput Assist Tomogr. 2005. 29:668–676.34. Smart SD, Firbank MJ, O'Brien JT. Validation of automated white matter hyperintensity segmentation. J Aging Res. 2011. 2011:391783.35. Admiraal-Behloul F, van den Heuvel DM, Olofsen H, van Osch MJ, van der Grond J, van Buchem MA, et al. Fully automatic segmentation of white matter hyperintensities in MR images of the elderly. Neuroimage. 2005. 28:607–617.
Article36. van den Heuvel DM, ten Dam VH, de Craen AJ, Admiraal-Behloul F, van Es AC, Palm WM, et al. Measuring longitudinal white matter changes: comparison of a visual rating scale with a volumetric measurement. AJNR Am J Neuroradiol. 2006. 27:875–878.
Article37. Kawata Y, Arimura H, Yamashita Y, Magome T, Ohki M, Toyofuku F, et al. Computer-aided evaluation method of white matter hyperintensities related to subcortical vascular dementia based on magnetic resonance imaging. Comput Med Imaging Graph. 2010. 34:370–376.
Article38. Jack CR Jr, O'Brien PC, Rettman DW, Shiung MM, Xu Y, Muthupillai R, et al. FLAIR histogram segmentation for measurement of leukoaraiosis volume. J Magn Reson Imaging. 2001. 14:668–676.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Association of Low Blood Pressure with White Matter Hyperintensities in Elderly Individuals with Controlled Hypertension
- Correlates of Depression and Anxiety in Acute Stroke Patients
- The Association of Cognitive Dysfunction with White Matter Hyperintensity in Alzheimer's Disease and Mild Cognitive Impairment
- Volumetric Brain MRI Study in Patients with First Episode Schizophrenia
- Relationship Between Cerebral Microbleeds and Aspirin Use Regarding White Matter Hyperintensity Volume