J Korean Ophthalmol Soc.
2015 Jan;56(1):138-141. 10.3341/jkos.2015.56.1.138.
Inadvertent Intralenticular Dexamethasone Implant for Diabetic Macular Edema Unresponsive to Bevacizumab
- Affiliations
-
- 1Department of Ophthalmology, Chuncheon Sacred Heart Hospital, Hallym University College of Medicine, Chuncheon, Korea. mcshin@hallym.ac.kr
- KMID: 2216168
- DOI: http://doi.org/10.3341/jkos.2015.56.1.138
Abstract
- PURPOSE
To report a case of inadvertent intralenticular slow-release dexamthasone implant (Ozurdex(R), Allergan Inc., Irvine, CA, USA) for diabetic macular edema unresponsive to bevacizumab.
CASE SUMMARY
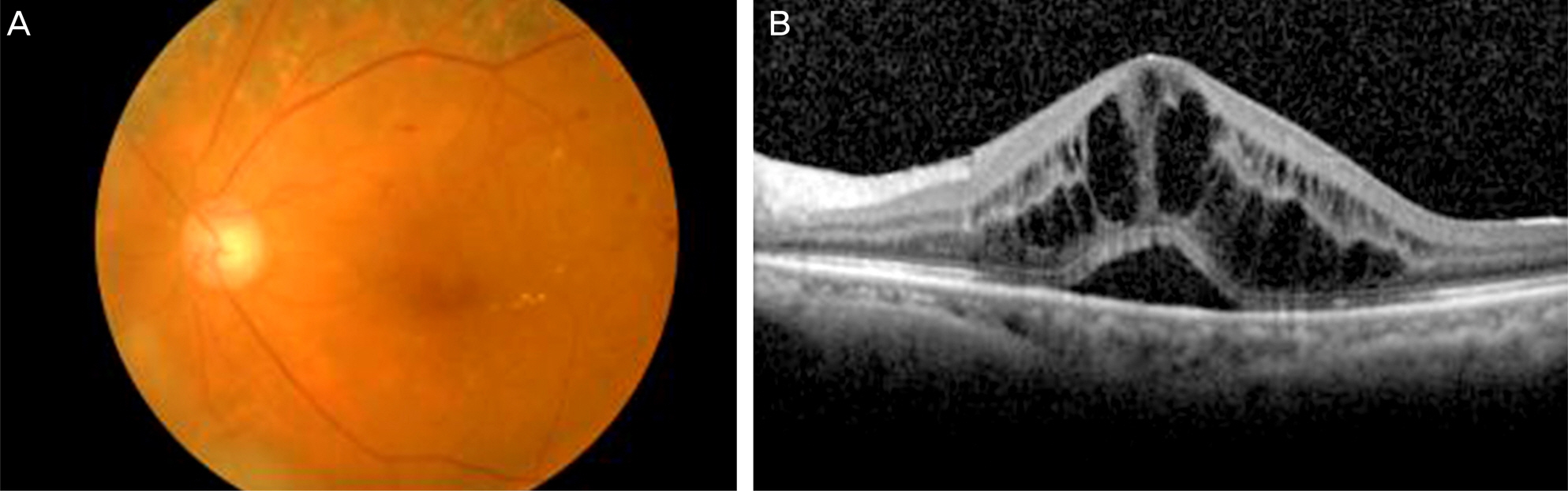
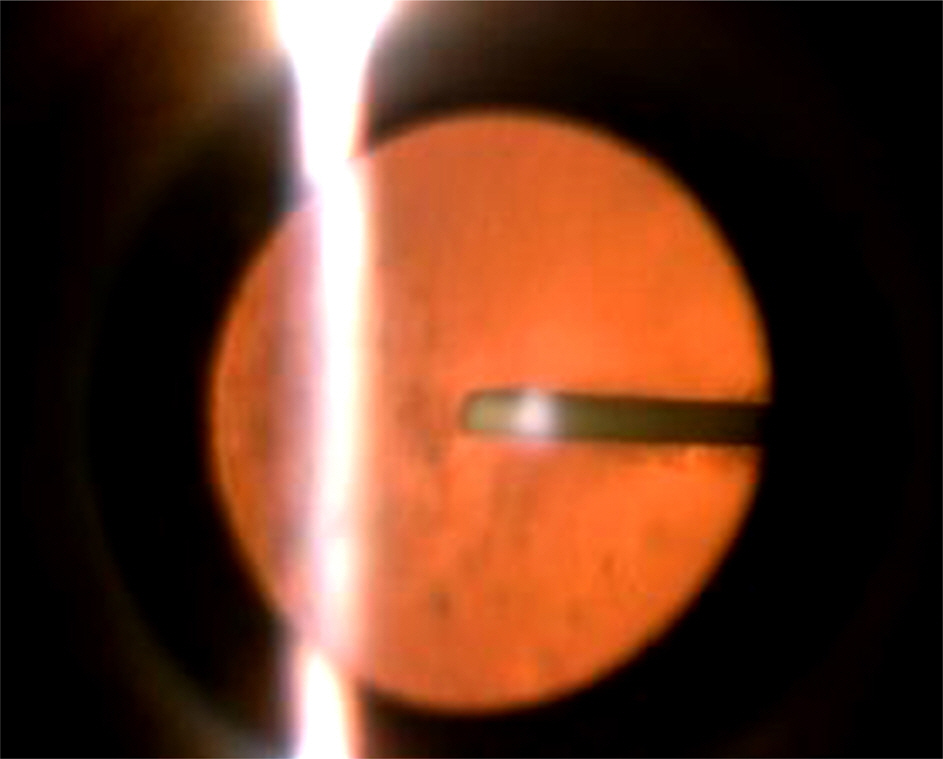
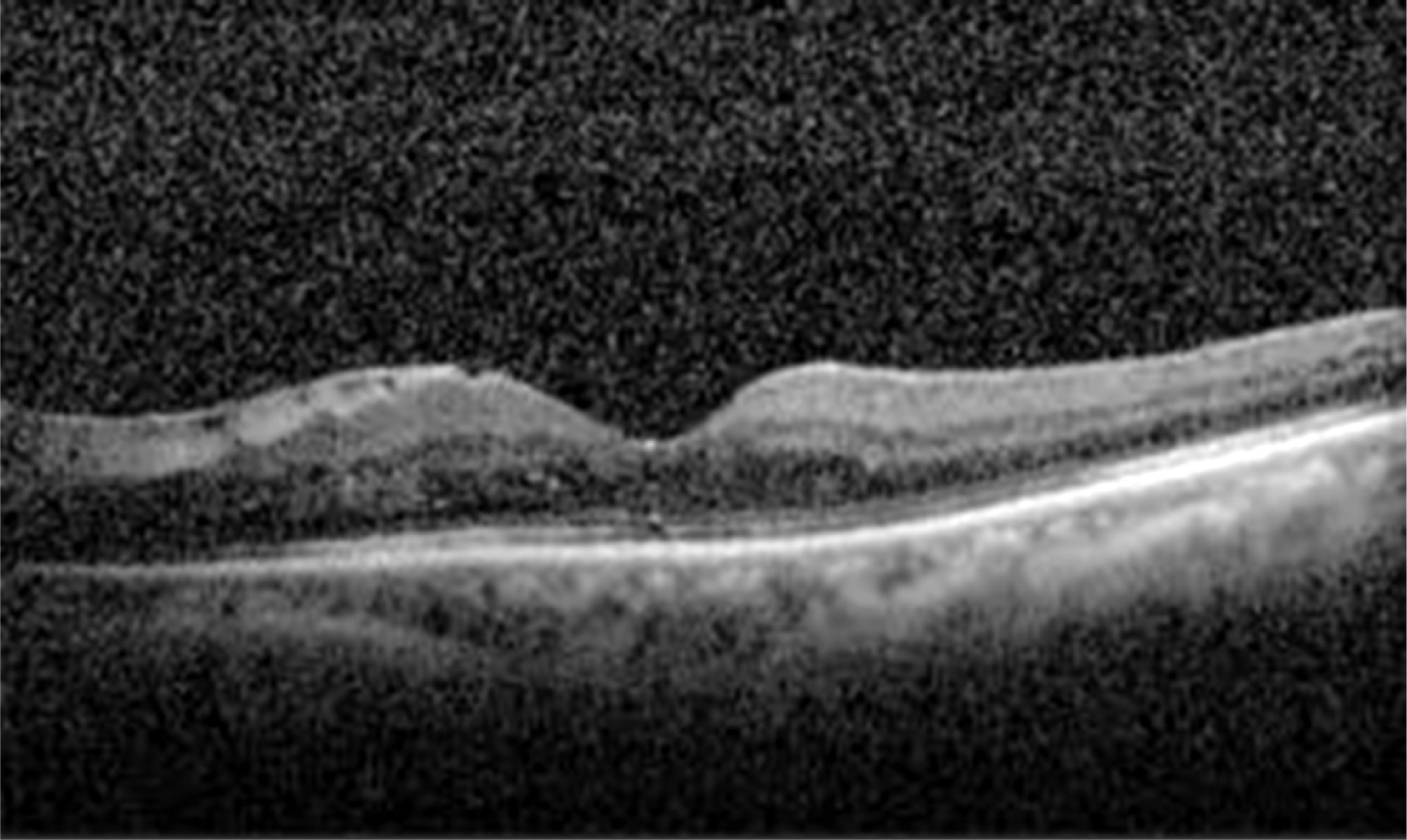
A 71-year-old woman presented with proliferative diabetic retinopathy. During follow-up, diabetic macular edema developed in both eyes and did not improve with intravitreal bevacizumab injections. For refractory diabetic macular edema, slow-release dexamthasone implant (Ozurdex(R)) was to be injected at the vitreous cavity of her left eye, but it was inadvertently injected into the crystalline lens. The patient was followed closely for 10 months. Diabetic macular edema completely resolved 1 month after the injection and did not recur during follow-up. There were no severe complications except mild cataract formation. Best-corrected visual acuity for the left eye improved from 0.1 to 0.2. The Ozurdex(R) implant slightly decreased after 10 months, but was still observed in the crystalline lens.
CONCLUSIONS
The inadvertent intralenticular dexamthasone implant was a rare complication but effective for diabetic macular edema.
MeSH Terms
Figure
Cited by 1 articles
-
A Case of Retinal Hemorrhage Following a Dexamethasone Intravitreal Implant
Min Hwan Kim, Young Hoon Lee
J Korean Ophthalmol Soc. 2017;58(1):93-97. doi: 10.3341/jkos.2017.58.1.93.
Reference
-
References
1. Sutter FK, Simpson JM, Gillies MC. Intravitreal triamcinolone for diabetic macular edema that persists after laser treatment: three-month efficacy and safety results of a prospective, random-ized, double-masked, placebo-controlled clinical trial. Ophthalmology. 2004; 111:2044–9.2. Vasconcelos-Santos DV, Nehemy PG, Schachat AP, Nehemy MB. Secondary ocular hypertension after intravitreal injection of 4 mg of triamcinolone acetonide: incidence and risk factors. Retina. 2008; 28:573–80.3. Chang-Lin JE, Attar M, Acheampong AA. . Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone in-travitreal implant. Invest Ophthalmol Vis Sci. 2011; 52:80–6.
Article4. Pardo-López D, Francés-Muñoz E, Gallego-Pinazo R, Díaz-Llopis M. Anterior chamber migration of dexametasone intravitreal implant (Ozurdex®). Graefes Arch Clin Exp Ophthalmol. 2012; 250:1703–4.5. Haller JA, Bandello F, Belfort R Jr. . Randomized, sham-con-trolled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010; 117:1134–46.e3.
Article6. Pacella E, Vestri AR, Muscella R. . Preliminary results of an in-travitreal dexamethasone implant (Ozurdex®) in patients with per-sistent diabetic macular edema. Clin Ophthalmol. 2013; 7:1423–8.7. Meyer CH, Rodrigues EB, Michels S. . Incidence of damage to the crystalline lens during intravitreal injections. J Ocul Pharmacol Ther. 2010; 26:491–5.
Article8. Rajak SN, Dubois VD, Mokete B, Casswell AG. The inadvertent administration of intralenticular triamcinolone. Eye (Lond). 2007; 21:426–7.
Article9. Kumar BV, Salvi SM, Prasad S. The inadvertent administration of intralenticular triamcinolone. Eye (Lond). 2007; 21:1428.
Article10. Koller S, Neuhann T, Neuhann I. [Conspicuous crystalline lens foreign body after intravitreal injection]. Ophthalmologe. 2012; 109:1119–21.11. Coca-Robinot J, Casco-Silva B, Armadá-Maresca F, García- Martínez J. Accidental injections of dexamethasone intravitreal implant (Ozurdex) into the crystalline lens. Eur J Ophthalmol. 2014; 24:633–6.
Article12. Karalezli A, Eroglu FC. Intravitreal dexamethasone implant in the crystalline lens. JCRS Online Case Reports. 2014; 2:e12–e15.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Short-Term Results of Dexamethasone Intravitreal Implant in Patients with Refractory Diabetic Macular Edema
- Delayed Symptoms after Intralenticular Dexamethasone Implant
- Comparison of Optical Coherence Tomography Biomarkers between Bevacizumab Good Responders and Nonresponders Who were Switched to Dexamethasone Implant in Diabetic Macular Edema
- Short-term Results of Intravitreal Dexamethasone Implant Combined with Bevacizumab versus Intravitreal Bevacizumab for Treatment-naive Diabetic Macular Edema
- A Multimodal Approach to Diabetic Macular Edema