J Korean Ophthalmol Soc.
2009 Aug;50(8):1221-1225. 10.3341/jkos.2009.50.8.1221.
Effectiveness of Preoperative Intravitreal Bevacizumab Injections in Pars Plana Vitrectomy for Proliferative Diabetic Retinopathy
- Affiliations
-
- 1Department of Ophthalmology, Kangnam Sacred Heart Hospital, College of Medicine, Hallym University, Seoul, Korea. ophkim@hallym.or.kr
- KMID: 2212538
- DOI: http://doi.org/10.3341/jkos.2009.50.8.1221
Abstract
- PURPOSE
To evaluate the efficacy of preoperative intravitreal bevacizumab (Avastin(R); Genetech, San Francisco, CA, USA) injections of pars plana vitrectomy (PPV) for proliferative diabetic retinopathy (PDR). METHODS: Thirty patients (30 eyes) who underwent PPV for treatment of PDR and received a preoperative intravitreal bevacizumab injection of 1.25 mg were retrospectively analyzed. The study group (group 1, 30 patients, 30 eyes) was compared with a control group (group 2, 29 patients, 30 eyes and matched with the study group for preoperative parameters) who underwent PPV without preoperative intravitreal bevacizumab injection. RESULTS: In both groups, visual acuity improved but there was no statistical significance. Intraoperative vitreous hemorrhage occurred in 14 eyes (46.7%) from group 1 and 11 eyes (36.7%) from group 2. There was no statistical significance of intraoperative bleeding occurrence (p=0.3). Postoperative vitreous hemorrhage occurred in 4 eyes from group 1 and 14 eyes from group 2. The group 1 had a lower incidence of postoperative hemorrhage than group 2 (p=0.005). CONCLUSIONS: Preoperative intravitreal bevacizumab injection appears effective in decreasing early postoperative vitreous hemorrhage and maybe technically helpful in PPV for PDR.
MeSH Terms
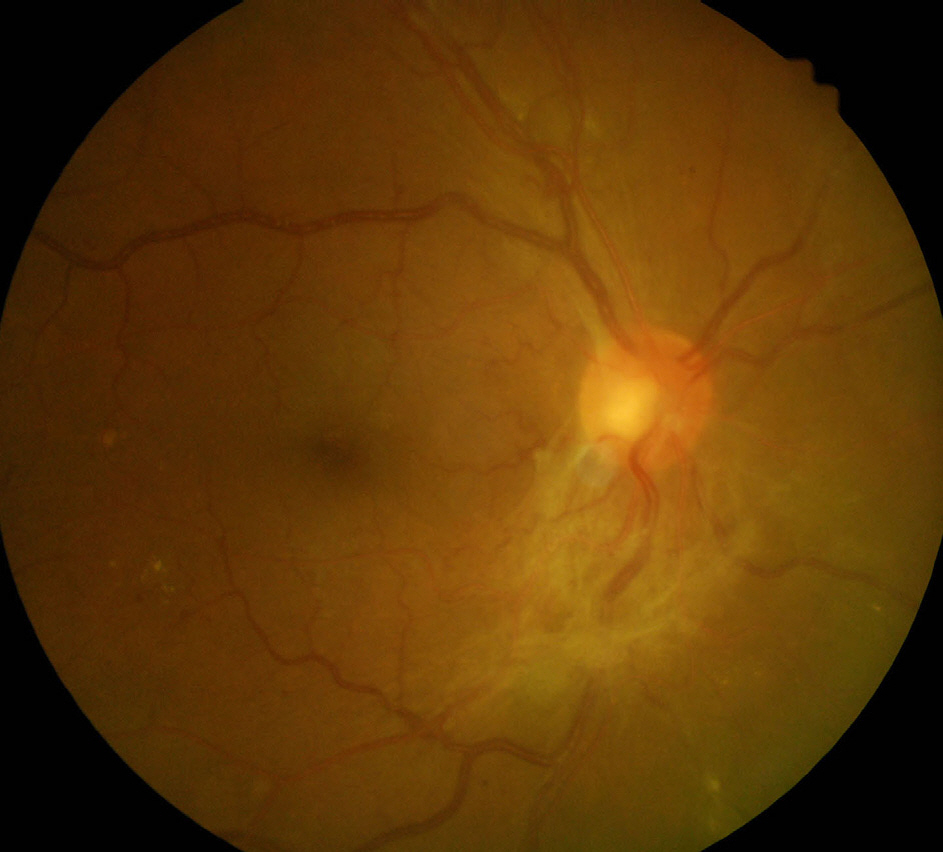
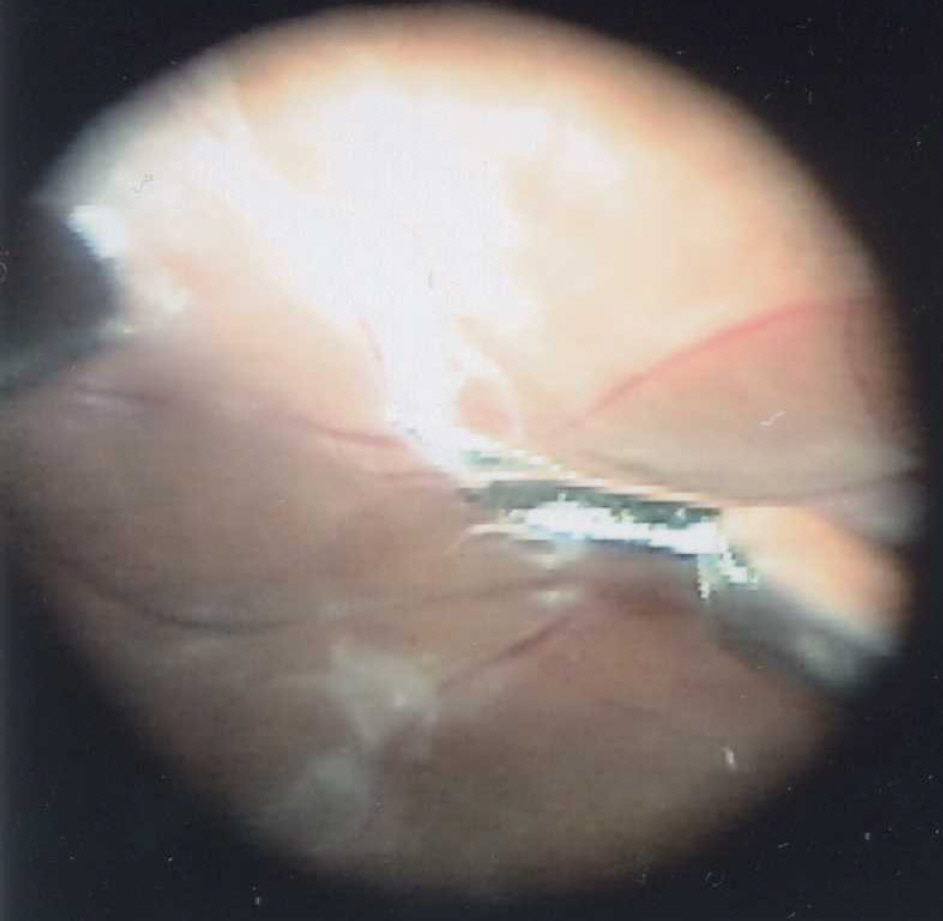
Figure
Cited by 1 articles
-
Effect of 23-gauge Sutureless Vitrectomy & Preoperative Bevacizumab on Results of Diabetic Vitrectomy
Dae Heon Han, Hee Jin Sohn, Dae Young Lee, Dong Heun Nam
J Korean Ophthalmol Soc. 2011;52(3):285-292. doi: 10.3341/jkos.2011.52.3.285.
Reference
-
References
1. Chen E, Park CH. Use Of Intravitreal bevacizumab as a preoperative adjunct for tractional retinal detachment repair in severe proliferative diabetic retinopathy. Retina. 2006; 26:699–700.
Article2. Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005; 36:331–5.
Article3. Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006; 113:363–72.
Article4. Avery RL. Regression of retinal and iris neovascularization after intravitreal bevacizumab (Avastin) treatment. Retina. 2006; 26:352–4.
Article5. Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006; 26:275–8.
Article6. Moss SE, Klein R, Klein BE. The 14-year incidence of visual loss in a diabetic population. Ophthalmology. 1998; 105:998–1003.
Article7. Rice TA, Michels RG. Long-term anatomic and functional results of vitrectomy for diabetic retinopathy. Am J Ophthalmol. 1980; 90:297–303.
Article8. Spaide RF, Fisher YL. Intravitreal bevacizumab (avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006; 26:275–8.
Article9. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004; 350:2335–42.
Article10. Schachat AP, Oyakawa RT, Michels RG, et al. Complications of vitreous surgery for diabetic retinopathy. II. Postoperative comp-lications. Ophthalmology. 1983; 90:522–30.11. Novak MA, Rice TA, Michels RG, Auer C. Vitreous hemorrhage after vitrectomy for diabetic retinopathy. Ophthalmology. 1984; 91:1485–9.
Article12. West JF, Gregor ZJ. Fibrovascular ingrowth and recurrent haemorrhage following diabetic vitrectomy. Br J Ophthalmol. 2000; 84:822–5.
Article13. Yang CM, Yeh PT, Yang CH. Intravitreal long-acting gas in the prevention of early postoperative vitreous hemorrhage in diabetic vitrectomy. Ophthalmology. 2007; 114:710–5.
Article14. Bhende M, Agraharam SG, Gopal L, et al. Ultrasound biomicro-scopy of sclerotomy sites after pars plana vitrectomy for diabetic vitreous hemorrhage. Ophthalmology. 2000; 107:1729–36.15. Hershberger VS, Augsburger JJ, Hutchins RK, et al. Fibro-vascular ingrowth at sclerotomy sites in vitrectomized diabetic eyes with recurrent vitreous hemorrhage: Ultrasound biomicro-scopy findings. Ophthalmology. 2004; 111:1215–21.16. Kreiger AE. Wound complications in pars plana vitrectomy. Retina. 1993; 13:335–44.
Article17. Yeh PT, Yang CM, Yang CH, Huang JS. Cryotherapy of the anterior retina and sclerotomy sites in diabetic vitrectomy to prevent recurrent vitreous hemorrhage. An ultrasound biomicro-scopy study. Ophthalmology. 2005; 112:2095–102.18. Yeoh J, Williams C, Allen P, et al. Avastin as an adjunct to vitrectomy in the management of severe proliferative diabetic retinopathy: a prospective case series. Clin Experiment Ophthalmol. 2008; 36:449–54.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of 23-gauge Sutureless Vitrectomy & Preoperative Bevacizumab on Results of Diabetic Vitrectomy
- The Clinical Evaluation of Pars Plana Vitrectomy in various Ocular Disease
- Vitreous Web after Pars Plana Vitrectomy and Bevacizumab Injection
- Efficacy of Intravitreal Triamcinolone Acetonide Injections at the End of Pars Plana Vitrectomy in Proliferative Diabetic Retinopathy
- Fibrin Sealant Injection for Control of Hemorrhage in Vitreous Surgery of Proliferative Diabetic Retinopathy