J Korean Surg Soc.
2013 Apr;84(4):238-244. 10.4174/jkss.2013.84.4.238.
Selective shunt during carotid endarterectomy using routine awake test with respect to a lower shunt rate
- Affiliations
-
- 1Division of Transplantation and Vascular Surgery, Department of Surgery, Kyungpook National University School of Medicine, Daegu, Korea. shuh@knu.ac.kr
- 2Department of Neurology, Kyungpook National University School of Medicine, Daegu, Korea.
- KMID: 2212469
- DOI: http://doi.org/10.4174/jkss.2013.84.4.238
Abstract
- PURPOSE
To evaluate shunt rate and discuss the resultsrelated to selective shunt placement during carotid endarterectomy (CEA) using routine awake test.
METHODS
Patients with CEA from 2007 to 2011 were retrospectively reviewed from prospectively collected data. The need for shunt placement was determined by the awake test, based on the alteration in the neurologic examination. We collected data by using the clinical records and imaging studies, and investigated factors related to selective shunt such as collateral circulation and contralateral internal carotid artery (ICA) stenosis.
RESULTS
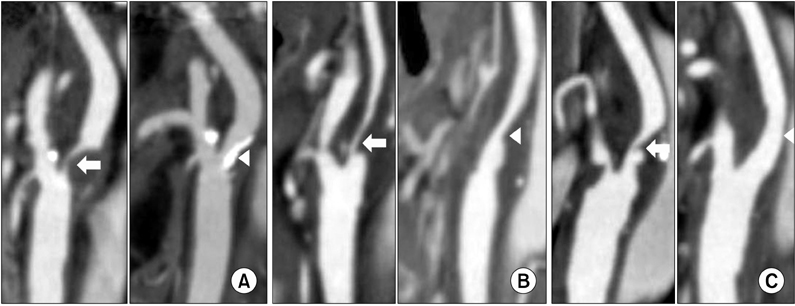
There were 45 CEAs under regional anesthesia with the awake test in 44 patients. The mean age was 61.8 +/- 7.1 years old. There were 82.2% (37/45) of males, and 68.9% (31/45) of symptomatic patients. Selective shunt placement had been performed in only two (4.4%) patients. Among them fewer cases (4%) had severe (stenosis >70%) contralateral ICA lesions, and more cases (91%) of complete morphology of the anterior or posterior circulation in the circle of Willis. There was no perioperative stroke, myocardial infarctionor death, and asymptomatic new brain lesions were detected in 4 patients (9%), including 2 cases of selective shunt placement.
CONCLUSION
CEA under routine awake test could besafe and feasible method with low shunt placement rate in selected patients.
MeSH Terms
Figure
Reference
-
1. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1991. 325:445–453.2. Frawley JE, Hicks RG, Gray LJ, Niesche JW. Carotid endarterectomy without a shunt for symptomatic lesions associated with contralateral severe stenosis or occlusion. J Vasc Surg. 1996. 23:421–427.3. Hertzer NR, O'Hara PJ, Mascha EJ, Krajewski LP, Sullivan TM, Beven EG. Early outcome assessment for 2228 consecutive carotid endarterectomy procedures: the Cleveland Clinic experience from 1989 to 1995. J Vasc Surg. 1997. 26:1–10.4. Lee KB, Lee KH, Chung CS, Kim GM, Byun HS, Jeon P, et al. Carotid endarterectomy: analysis of early complications (<30 days) and risk factors for postoperative new brain infarction. J Korean Surg Soc. 2009. 77:195–201.5. Gumerlock MK, Neuwelt EA. Carotid endarterectomy: to shunt or not to shunt. Stroke. 1988. 19:1485–1490.6. Sundt TM Jr, Sharbrough FW, Piepgras DG, Kearns TP, Messick JM Jr, O'Fallon WM. Correlation of cerebral blood flow and electroencephalographic changes during carotid endarterectomy: with results of surgery and hemodynamics of cerebral ischemia. Mayo Clin Proc. 1981. 56:533–543.7. Calligaro KD, Dougherty MJ. Correlation of carotid artery stump pressure and neurologic changes during 474 carotid endarterectomies performed in awake patients. J Vasc Surg. 2005. 42:684–689.8. Hans SS, Jareunpoon O. Prospective evaluation of electroencephalography, carotid artery stump pressure, and neurologic changes during 314 consecutive carotid endarterectomies performed in awake patients. J Vasc Surg. 2007. 45:511–515.9. Wober C, Zeitlhofer J, Asenbaum S, Claeys L, Czerny M, Wolfl G, et al. Monitoring of median nerve somatosensory evoked potentials in carotid surgery. J Clin Neurophysiol. 1998. 15:429–438.10. Belardi P, Lucertini G, Ermirio D. Stump pressure and transcranial Doppler for predicting shunting in carotid endarterectomy. Eur J Vasc Endovasc Surg. 2003. 25:164–167.11. Friedell ML, Clark JM, Graham DA, Isley MR, Zhang XF. Cerebral oximetry does not correlate with electroencephalography and somatosensory evoked potentials in determining the need for shunting during carotid endarterectomy. J Vasc Surg. 2008. 48:601–606.12. Illig KA, Sternbach Y, Zhang R, Burchfiel J, Shortell CK, Rhodes JM, et al. EEG changes during awake carotid endarterectomy. Ann Vasc Surg. 2002. 16:6–11.13. Lawrence PF, Alves JC, Jicha D, Bhirangi K, Dobrin PB. Incidence, timing, and causes of cerebral ischemia during carotid endarterectomy with regional anesthesia. J Vasc Surg. 1998. 27:329–334.14. McCarthy RJ, Walker R, McAteer P, Budd JS, Horrocks M. Patient and hospital benefits of local anaesthesia for carotid endarterectomy. Eur J Vasc Endovasc Surg. 2001. 22:13–18.15. McCleary AJ, Maritati G, Gough MJ. Carotid endarterectomy; local or general anaesthesia? Eur J Vasc Endovasc Surg. 2001. 22:1–12.16. Aburahma AF, Stone PA, Hass SM, Dean LS, Habib J, Keiffer T, et al. Prospective randomized trial of routine versus selective shunting in carotid endarterectomy based on stump pressure. J Vasc Surg. 2010. 51:1133–1138.17. Tan TW, Garcia-Toca M, Marcaccio EJ Jr, Carney WI Jr, Machan JT, Slaiby JM. Predictors of shunt during carotid endarterectomy with routine electroencephalography monitoring. J Vasc Surg. 2009. 49:1374–1378.18. Lee JH, Choi CG, Kim DK, Kim GE, Lee HK, Suh DC. Relationship between circle of Willis morphology on 3D time-of-flight MR angiograms and transient ischemia during vascular clamping of the internal carotid artery during carotid endarterectomy. AJNR Am J Neuroradiol. 2004. 25:558–564.19. Hartkamp MJ, van Der Grond J, van Everdingen KJ, Hillen B, Mali WP. Circle of Willis collateral flow investigated by magnetic resonance angiography. Stroke. 1999. 30:2671–2678.20. Schneider JR, Droste JS, Schindler N, Golan JF, Bernstein LP, Rosenberg RS. Carotid endarterectomy with routine electroencephalography and selective shunting: Influence of contralateral internal carotid artery occlusion and utility in prevention of perioperative strokes. J Vasc Surg. 2002. 35:1114–1122.21. Hendrikse J, Rutgers DR, Klijn CJ, Eikelboom BC, van der Grond J. Effect of carotid endarterectomy on primary collateral blood flow in patients with severe carotid artery lesions. Stroke. 2003. 34:1650–1654.22. Sternbach Y, Illig KA, Zhang R, Shortell CK, Rhodes JM, Davies MG, et al. Hemodynamic benefits of regional anesthesia for carotid endarterectomy. J Vasc Surg. 2002. 35:333–339.23. Aleksic M, Luebke T, Brunkwall J. Outcome of carotid endarterectomy under local anaesthesia with respect to the patients' risk profile. Vasa. 2009. 38:225–233.24. Palombo D, Lucertini G, Mambrini S, Zettin M. Subtle cerebral damage after shunting vs non shunting during carotid endarterectomy. Eur J Vasc Endovasc Surg. 2007. 34:546–551.25. Rerkasem K, Rothwell PM. Routine or selective carotid artery shunting for carotid endarterectomy and different methods of monitoring in selective shunting. Stroke. 2010. 41:e53–e54.26. Schnaudigel S, Groschel K, Pilgram SM, Kastrup A. New brain lesions after carotid stenting versus carotid endarterectomy: a systematic review of the literature. Stroke. 2008. 39:1911–1919.27. Bonati LH, Jongen LM, Haller S, Flach HZ, Dobson J, Nederkoorn PJ, et al. New ischaemic brain lesions on MRI after stenting or endarterectomy for symptomatic carotid stenosis: a substudy of the International Carotid Stenting Study (ICSS). Lancet Neurol. 2010. 9:353–362.28. Lacroix V, Hammer F, Astarci P, Duprez T, Grandin C, Cosnard G, et al. Ischemic cerebral lesions after carotid surgery and carotid stenting. Eur J Vasc Endovasc Surg. 2007. 33:430–435.29. de Borst GJ, Moll FL, van de Pavoordt HD, Mauser HW, Kelder JC, Ackerstaf RG. Stroke from carotid endarterectomy: when and how to reduce perioperative stroke rate? Eur J Vasc Endovasc Surg. 2001. 21:484–489.30. Bouri S, Thapar A, Shalhoub J, Jayasooriya G, Fernando A, Franklin IJ, et al. Hypertension and the post-carotid endarterectomy cerebral hyperperfusion syndrome. Eur J Vasc Endovasc Surg. 2011. 41:229–237.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dual Monitoring with Stump Pressure and Electroencephalography During Carotid Endarterectomy
- Anesthetic Management of Carotid Endarterectomy under Cervical Plexus Block
- Intraoperative Electroencephalographic Changes During Carotid Endarterectomy
- Near-Infrared Spectroscopy versus Transcranial Doppler-Based Monitoring in Carotid Endarterectomy
- Eversion Carotid Endarterectomy : A Short Review