J Korean Ophthalmol Soc.
2009 Apr;50(4):551-557. 10.3341/jkos.2009.50.4.551.
Preferential Hyperacuity Perimeter in Exudative AMD With Pigment Epithelial Detachment
- Affiliations
-
- 1Department of Ophthalmology, Kangwon National University, School of Medicine, Chuncheon, Korea. opticus@kangwon.ac.kr
- KMID: 2212256
- DOI: http://doi.org/10.3341/jkos.2009.50.4.551
Abstract
-
PURPOSE: To analyze the hyperacuity defects by preferential hyperacuity perimeter (PHP) in exudative age-related macular degeneration (AMD) and correlate them with the properties of pigment epithelial detachment (PED) obtained by optical coherence tomography (OCT).
METHODS
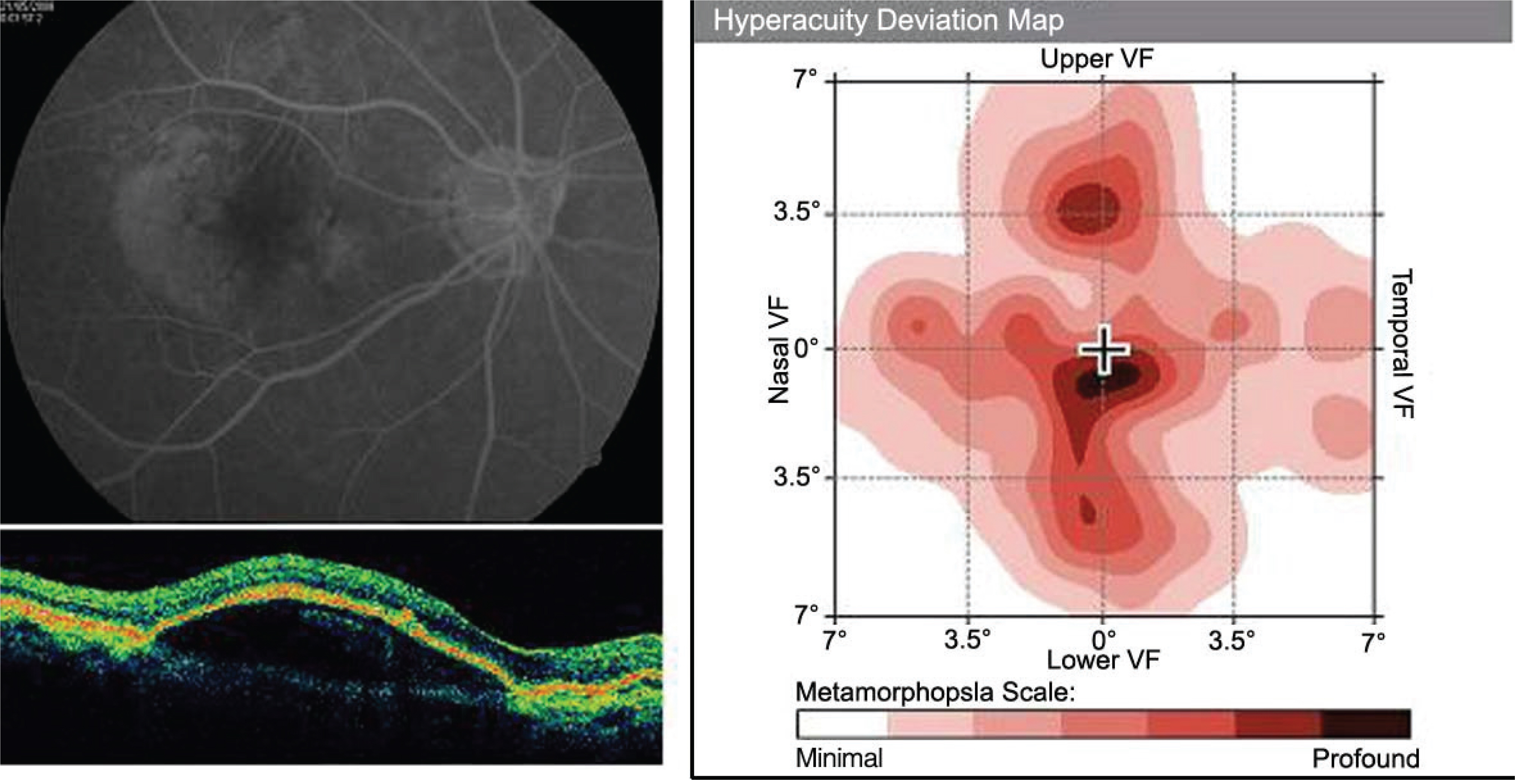
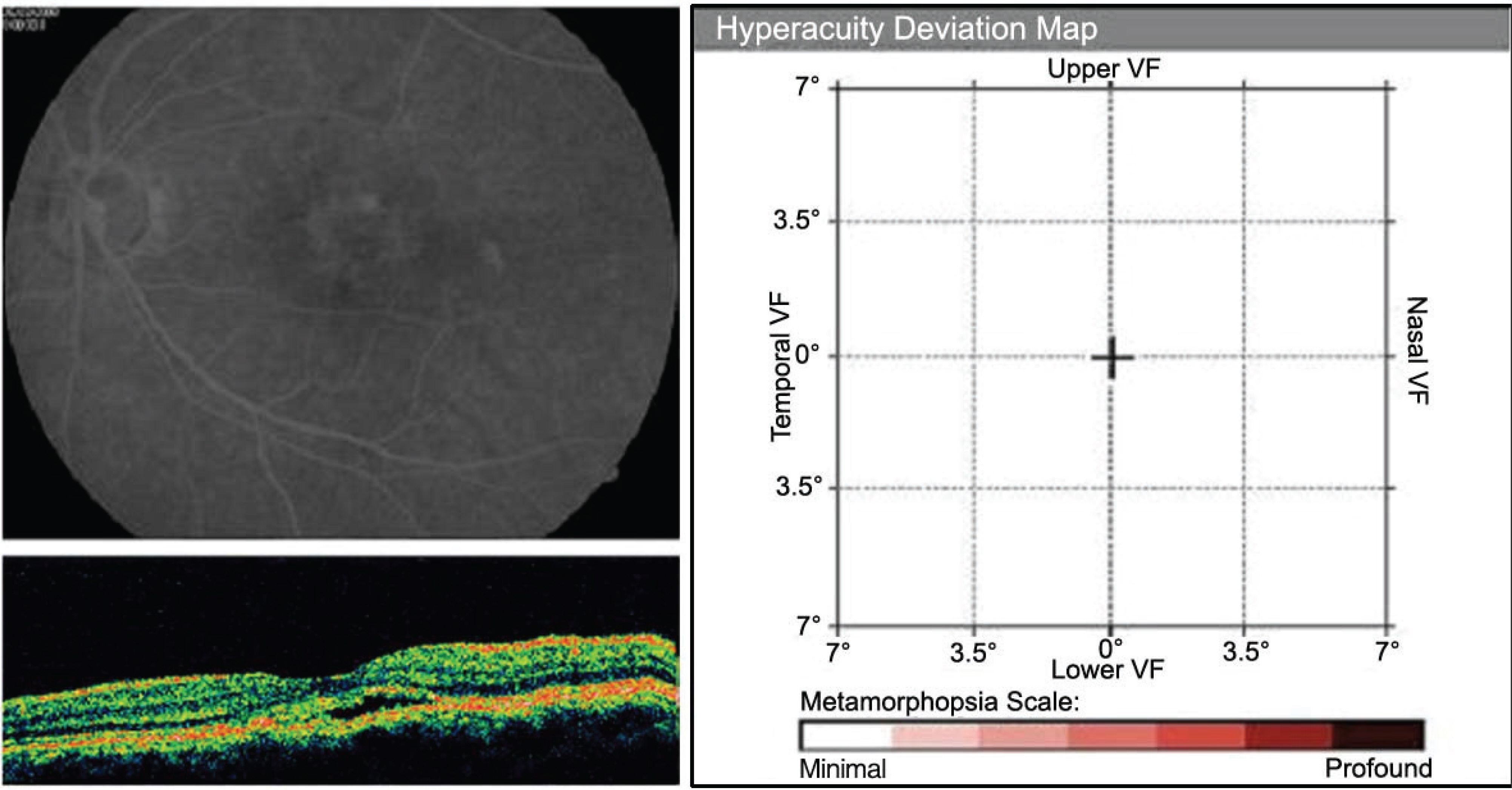
Thirty eyes with exudative AMD with choroidal neovascularization (CNV) diagnosed by fluorescein angiography underwent PHP for hyperacuity defect and OCT for PED length and height. We compared hyperacuity defect with the shape of the PED by OCT.
RESULTS
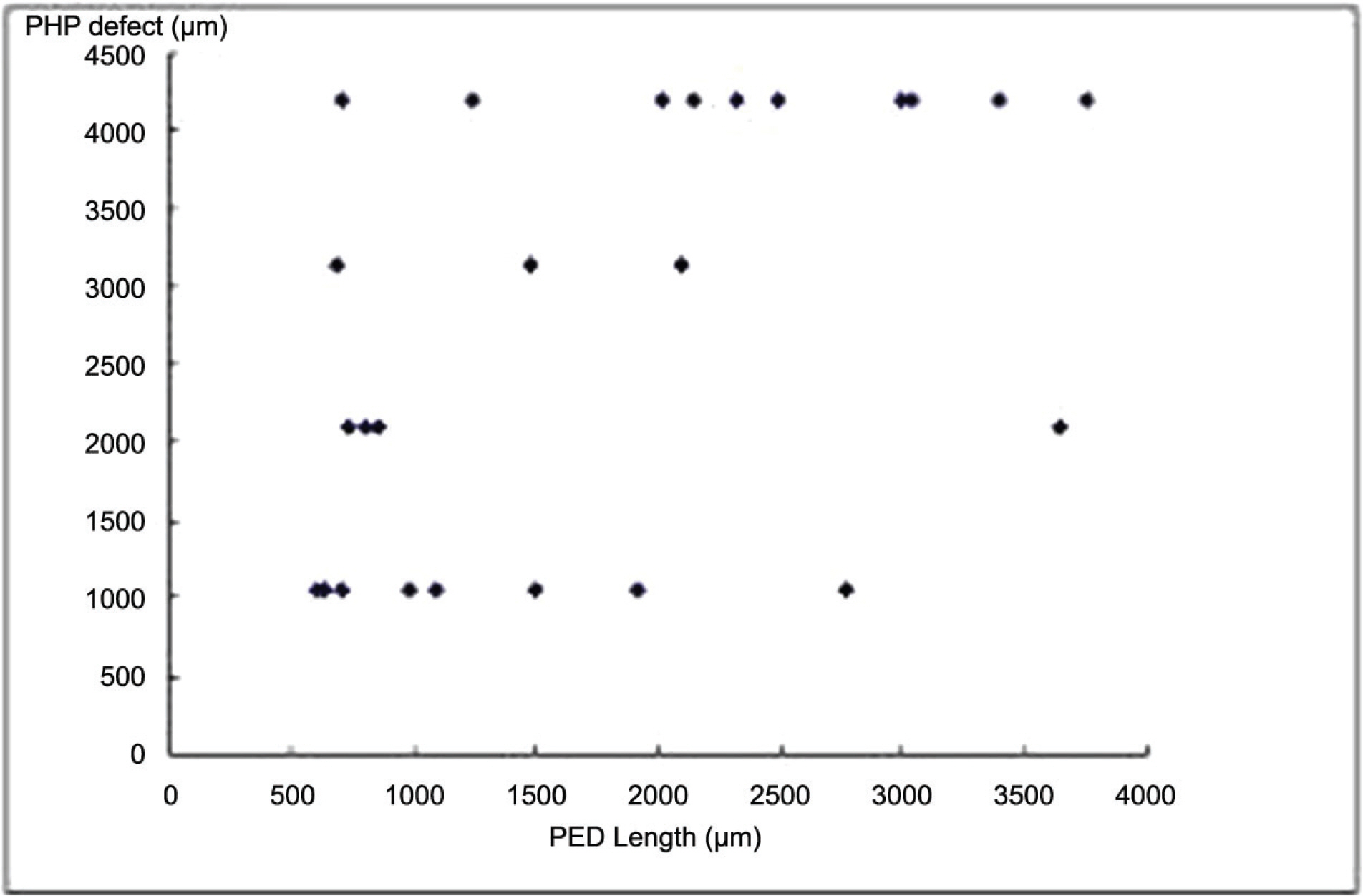
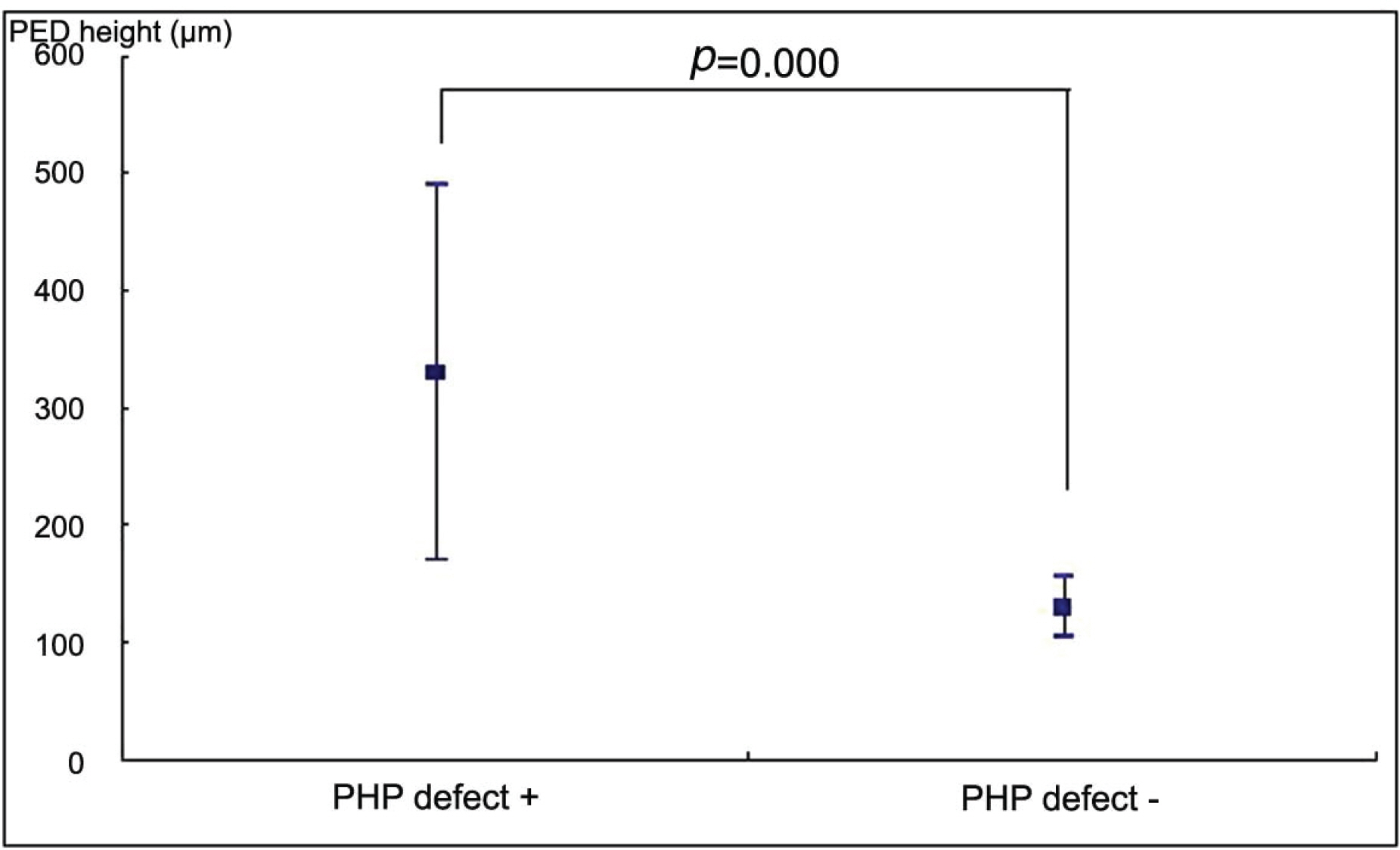
26 eyes with exudative AMD with CNV tested positive for hyperacuity defects. The size of the hyperacuity defect by PHP and the PED length by OCT showed positive correlation (p=0.010). In the 4 eyes that tested negative for hyperacuity defects, the PED was not high although the size was large.
CONCLUSIONS
Our results suggest that PHP is a useful method to detect a change of pigment epithelial layer in AMD and the presences of a hyperacuity defect is more sensitive for PED height than size. These results suggest that PHP is useful to detect the state and the activity of CNV lesion.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992; 99:933–43.2. Pizzarello LD. The dimensions of the problem of eye disease among the elderly. Ophthalmology. 1987; 94:1191–5.
Article3. Bressler NM, Bressler SB. Preventive ophthalmology. Age-related macular degeneration. Ophthalmology. 1995; 102:1206–11.4. Macular Photocoagulation Study Group. Argon laser photocoagulation for neovascular maculopathy. Five-year results from randomized clinical trials. Arch Ophthalmol. 1991; 109:1109–14.5. Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal neovascular lesions of age-related macular degeneration. Updated findings from two clinical trials. Arch Ophthalmol. 1993; 111:1200–9.6. Macular Photocoagulation Study Group. Laser photocoagulation for juxtafoveal choroidal neovascularization. Five-year results from randomized clinical trials. Arch Ophthalmol. 1994; 112:500–9.7. Achard OA, Safran AB, Durect FC, Ragama E. Role of the completion phenomenon in the evaluation of Amsler grid results. Am J Ophthalmol. 1995; 120:322–9.
Article8. Parkes L, Lund J, Angelucci A, et al. Compulsary averaging of crowded orientation signals in human vision. Nat Neurosci. 2001; 4:739–44.9. Loewenstein A, Malach R, Goldstein M, et al. Replacing theamsler grid: A new method for monitoring patients with age-related macular degeneration. Ophthalmology. 2003; 110:966–70.10. Westheimer G. Visual acuity and hyperacuity: resolution, localization, form. Am J Optom Physiol Opt. 1987; 64:567–74.11. Lakshminarayanan V, Aziz S, Enoch JM. Variation of the hyperacuity gap function with age. Optom Vis Sci. 1992; 69:423–6.
Article12. Wang YZ. Effects of aging on shape discrimination. Optom Vis Sci. 2001; 78:447–54.
Article13. Kleine DW, Culham JC, Bartel P, et al. Aging effects on Vernier hyperacuity: a function of ocillation rate but not target contrast. Optom Vis Sci. 2001; 78:676–82.14. Enoch JM, William RA, Essock EA, et al. Hyperacuity perimetry. Assessment of macular function through ocular opacities. Arch Ophthalmol. 1984; 102:1164–8.15. Age-Related Eye Disease Study Reaserch Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss. AREDS report no. 8. Arch Ophthalmol. 2001; 119:1417–36.16. Vander JF, Morgan CM, Schatz H. Growth rate of subretinal neovascularization in age-related macular degeneration. Ophthalmology. 1989; 96:1422–6.
Article17. Klein ML, Jorizzo PA, Watzke RC. Growth feature of choroidal neovascularization membranes in age-related macular degeneration. Ophthalmology. 1989; 96:1416–9.18. Bressler NM, Frost LA, Bressler SB, et al. Natural course of poorly defined choroidal neovascularization associated with macular degeneration. Arch Ophthalmol. 1988; 106:1537–42.
Article19. Loewenstein A, Malach R, Goldstein M, et al. Replacing theamsler grid: A new method for monitoring patients withage-related macular degeneration. Ophthalmology. 2003; 110:966–70.20. Preferential Hyperacuity perimeter (PHP) research group. Results of a multicenter clinical trial to evaluate the preferential hyperacuity perimeter for detection of age-related maculardegene-ration. Retina. 2005; 25:296–303.21. Preferential Hyperacuity perimeter (PHP) research group. Pre-ferential Hyperacuity Perimeter (PreView PHP) for detecting Choroidal Neovascularization Study. Ophthalmology. 2005; 112:1758–65.22. Sarks S, Cherepanoff S, Killingsworth M, Sarks J. Relationship of Basal laminar deposit and membranous debris to the clinical presentation of early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007; 48:968–77.
Article23. Schlingemann RO. Role of growth factors and the wound healing response in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2004; 242:91–101.
Article24. Hera R, Keramidas M, Mouillon M, et al. Expression of VEGF and angiopoietins in subfoveal membranes from patients with age-related macular degeneration. Am J Ophthalmol. 2005; 139:589–96.
Article25. Tong JP, Yao YF. Contribution of VEGF and PEDF to choroidal angiogenesis: a need for balanced expressions. Clin Biochem. 2006; 39:267–76.
Article26. Giovannini A, Amato GP, Mariotti C, et al. OCT imaging of choroidal neovascularisation and its role in the determination of patients' eligibility for surgery. Br J Ophthalmol. 1999; 83:438–42.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlation of OCT and Preferential Hyperacuity Perimeter in Exudative Age-related Macular Degeneration
- Preferential Hyperacuity Perimeter (PHP) in Myopic CNV
- Analysis of Preferential Hyperacuity Perimeter Results in Maculopathy Caused by Various Retinal Diseases
- An Analysis of Metamorphopsia Using Preferential Hyperacuity Perimeter Following Macular-off RRD Surgical Repair
- Angle-closure Attack after Retinal Pigment Epithelium Double-tear and Hemorrhagic Retinal Detachment in Exudative Macular Degeneration