J Korean Surg Soc.
2010 Aug;79(2):94-102. 10.4174/jkss.2010.79.2.94.
Overexpression of p53, Mutation of hMLH1 and Microsatellite Instability in Gastric Carcinomas: Clinicopathologic Implications and Prognosis
- Affiliations
-
- 1Department of Surgery, Seoul National University Bundang Hospital, Seongnam, Korea. hhkim@snubh.org
- 2Department of Pathology, Seoul National University Bundang Hospital, Seongnam, Korea.
- 3Department of Surgery, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Surgery, Dankook University Hospital, Seoul, Korea.
- KMID: 2211978
- DOI: http://doi.org/10.4174/jkss.2010.79.2.94
Abstract
- PURPOSE
Mutated p53 is a tumor suppressor gene, hMLH1 is a mismatch repair gene, and hypermethylation of hMLH1 follows microsatellite instability (MSI). This research's aim is to investigate mutated p53, inactivated hMLH1 and MSI in gastric cancer and their clinicopathologic implications.
METHODS
Between 2003 and 2007, 618 patients underwent curative radical gastrectomy for gastric cancer at Seoul National University Bundang Hospital in Korea. We reviewed their medical charts and the pathologic reports with immunohistochemistry for p53, hMLH1 and polymerase chain reaction for MSI in 509, 499, and 561 cases, respectively. These genetic markers were statistically compared with clinicopathologic features and postoperative survival.
RESULTS
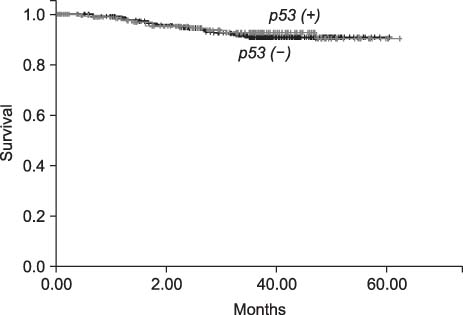
The expression ratios of mutated p53, inactivated hMLH1, and MSI were 32.8%, 8.4%, and 8.7%, respectively. Mutation of p53 occurred more frequently in aged group (over 40), differentiated group (against the non-differentiated group), intestinal type, infiltrative type and positive lymph node metastasis group. Inactivated hMLH1 occurred more frequently in aged group, differentiated group, intestinal type and expanding growth type group. MSI was found more frequently in aged group, intestinal type and expanding growth type group. All three genetic markers had no significant associations with the 5-year survival.
CONCLUSION
We identified significant relationships between mutated p53, inactivated hMLH1, and MSI with some clinicopathologic features of gastric cancer. However, there were no apparent relationships between p53, hMLH1, and MSI and prognosis.
Keyword
MeSH Terms
Figure
Reference
-
1. Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992. 52:6735–6740.2. Tahara E. Molecular mechanism of stomach carcinogenesis. J Cancer Res Clin Oncol. 1993. 119:265–272.3. Harris AL. Mutant p53--the commonest genetic abnormality in human cancer? J Pathol. 1990. 162:5–6.4. Hollstein MC, Peri L, Mandard AM, Welsh JA, Montesano R, Metcalf RA, et al. Genetic analysis of human esophageal tumors from two high incidence geographic areas: frequent p53 base substitutions and absence of ras mutations. Cancer Res. 1991. 51:4102–4106.5. Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991. 352:345–347.6. Woerner SM, Kloor M, von Knebel Doeberitz M, Gebert JF. Microsatellite instability in the development of DNA mismatch repair deficient tumors. Cancer Biomark. 2006. 2:69–86.7. Thibodeau SN, French AJ, Roche PC, Cunningham JM, Tester DJ, Lindor NM, et al. Altered expression of hMSH2 and hMLH1 in tumors with microsatellite instability and genetic alterations in mismatch repair genes. Cancer Res. 1996. 56:4836–4840.8. Fleisher AS, Esteller M, Wang S, Tamura G, Suzuki H, Yin J, et al. Hypermethylation of the hMLH1 gene promoter in human gastric cancers with microsatellite instability. Cancer Res. 1999. 59:1090–1095.9. Rhyu MG, Park WS, Meltzer SJ. Microsatellite instability occurs frequently in human gastric carcinoma. Oncogene. 1994. 9:29–32.10. Peddanna N, Holt S, Verma RS. Genetics of gastric cancer. Anticancer Res. 1995. 15:2055–2064.11. Correa P, Shiao YH. Phenotypic and genotypic events in gastric carcinogenesis. Cancer Res. 1994. 54:7 Suppl. 1941s–1943s.12. Starzynska T, Markiewski M, Domagala W, Marlicz K, Mietkiewski J, Roberts SA, et al. The clinical significance of p53 accumulation in gastric carcinoma. Cancer. 1996. 77:2005–2012.13. Martin HM, Filipe MI, Morris RW, Lane DP, Silvestre F. p53 expression and prognosis in gastric carcinoma. Int J Cancer. 1992. 50:859–862.14. Ku KB, Park SH, Chung HY, Yu W, Lee MH. p53 Mutation and p53 protein expression in gastric cancer tissue. J Korean Surg Soc. 2007. 72:283–289.15. Rugge M, Shiao YH, Busatto G, Cassaro M, Strobbe C, Russo VM, et al. The p53 gene in patients under the age of 40 with gastric cancer: mutation rates are low but are associated with a cardiac location. Mol Pathol. 2000. 53:207–210.16. Uchino S, Noguchi M, Ochiai A, Saito T, Kobayashi M, Hirohashi S. p53 mutation in gastric cancer: a genetic model for carcinogenesis is common to gastric and colorectal cancer. Int J Cancer. 1993. 54:759–764.17. Monig SP, Eidt S, Zirbes TK, Stippel D, Baldus SE, Pichlmaier H. p53 expression in gastric cancer: clinicopathological correlation and prognostic significance. Dig Dis Sci. 1997. 42:2463–2467.18. Lee HS, Choi SI, Lee HK, Kim HS, Yang HK, Kang GH, et al. Distinct clinical features and outcomes of gastric cancers with microsatellite instability. Mod Pathol. 2002. 15:632–640.19. Liu P, Zhang XY, Shao Y, Zhang DF. Microsatellite instability in gastric cancer and pre-cancerous lesions. World J Gastroenterol. 2005. 11:4904–4907.20. Oh SH, Choi YK, Hong KH, Kim SH, Paik NH, Yang YI, et al. Microsatellite instability and overexpression of p53 protein in human gastric carcinomas: clinicopathologic implications and prognosis. J Korean Surg Soc. 2000. 59:206–222.21. Leung SY, Yuen ST, Chung LP, Chu KM, Chan AS, Ho JC. hMLH1 promoter methylation and lack of hMLH1 expression in sporadic gastric carcinomas with high-frequency microsatellite instability. Cancer Res. 1999. 59:159–164.22. Samowitz WS, Holden JA, Curtin K, Edwards SL, Walker AR, Lin HA, et al. Inverse relationship between microsatellite instability and K-ras and p53 gene alterations in colon cancer. Am J Pathol. 2001. 158:1517–1524.23. Wu MS, Lee CW, Shun CT, Wang HP, Lee WJ, Chang MC, et al. Distinct clinicopathologic and genetic profiles in sporadic gastric cancer with different mutator phenotypes. Genes Chromosomes Cancer. 2000. 27:403–411.24. Seruca R, Santos NR, David L, Constancia M, Barroca H, Carneiro F, et al. Sporadic gastric carcinomas with microsatellite instability display a particular clinicopathologic profile. Int J Cancer. 1995. 64:32–36.25. Tamura G, Sakata K, Maesawa C, Suzuki Y, Terashima M, Satoh K, et al. Microsatellite alterations in adenoma and differentiated adenocarcinoma of the stomach. Cancer Res. 1995. 55:1933–1936.26. Nakajima T, Akiyama Y, Shiraishi J, Arai T, Yanagisawa Y, Ara M, et al. Age-related hypermethylation of the hMLH1 promoter in gastric cancers. Int J Cancer. 2001. 94:208–211.27. dos Santos NR, Seruca R, Constancia M, Seixas M, Sobrinho-Simoes M. Microsatellite instability at multiple loci in gastric carcinoma: clinicopathologic implications and prognosis. Gastroenterology. 1996. 110:38–44.28. Bodmer W, Bishop T, Karran P. Genetic steps in colorectal cancer. Nat Genet. 1994. 6:217–219.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Microsatellite Instability and Promoter Methylation of hMLH1 in Sporadic Gastric Carcinoma
- Microsatellite Instability and Overexpression of p53 Protein in Human Gastric Carcinomas: Clinicopathologic Implications and Prognosis
- Mutation of the Chk1 Gene in Gastric Cancers with Microsatellite Instability
- hMLH1/hMSH2 Protein Expression in Sporadic Colorectal Carcinoma and Its Clinicopathological Significance
- Microsatellite Instability in Gastric Adenocarcinoma Tissue Obtained by Endoscopic Biopsy