J Nutr Health.
2015 Aug;48(4):319-326. 10.4163/jnh.2015.48.4.319.
Comparative effect of dietary borage oil and safflower oil on anti-proliferation and ceramide metabolism in the epidermis of essential fatty acid deficient guinea pigs
- Affiliations
-
- 1Department of Medical Nutrition, Graduate School of East-West Medical Science, Kyung Hee University, Gyeonggi 446-701, Korea. choyunhi@khu.ac.kr
- KMID: 2209898
- DOI: http://doi.org/10.4163/jnh.2015.48.4.319
Abstract
- PURPOSE
Borage oil (BO) and safflower oil (SO) are efficacious in reversing epidermal hyperproliferation, which is caused by the disruption of epidermal barrier. In this study, we compared the antiproliferative effect of dietary BO and SO. Altered metabolism of ceramide (Cer), the major lipid of epidermal barrier, was further determined by measurement of epidermal levels of individual Cer, glucosylceramide (GlcCer), and sphingomyelin (SM) species, and protein expression of Cer metabolizing enzymes.
METHODS
Epidermal hyperproliferation was induced in guinea pigs by a hydrogenated coconut diet (HCO) for 8 weeks. Subsequently, animals were fed diets of either BO (group HCO + BO) or SO (group HCO + SO) for 2 weeks. As controls, animals were fed BO (group BO) or HCO (group HCO) diets for 10 weeks.
RESULTS
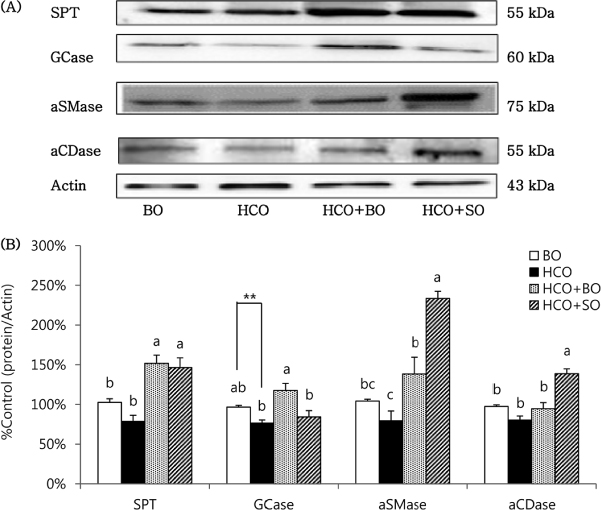
Epidermal hyperproliferation was reversed in groups HCO + BO (67.6% of group HCO) and HCO + SO (84.5% of group HCO). Epidermal levels of Cer1/2, GlcCer-A/B, and beta-glucocerebrosidase (GCase), an enzyme of GlcCer hydrolysis for Cer generation, were higher in group HCO + BO than in group HCO, and increased to levels similar to those of group BO. In addition, epidermal levels of SM1, serine palmitoyltransferase (SPT), and acidic sphingomyelinase (aSMase), enzymes of de novo Cer synthesis and SM hydrolysis for Cer generation, but not of Cer3-7, were higher in group HCO + BO than in group HCO. Despite an increase of SPT and aSMase in group HCO + SO to levels higher than in group HCO, epidermal levels of Cer1-7, GlcCer-A/B, and GCase were similar in these two groups. Notably, acidic ceramidase, an enzyme of Cer degradation, was highly expressed in group HCO + SO. Epidermal levels of GlcCer-C/D and SM-2/3 did not differ among groups.
CONCLUSION
Dietary BO was more prominent for reversing epidermal hyperproliferation by enhancing Cer metabolism with increased levels of Cer1/2, GlcCer-A/B, and SM1 species, and of GCase proteins.
MeSH Terms
-
Animals
Borago*
Carthamus tinctorius*
Ceramidases
Cocos
Diet
Epidermis*
Glucosylceramidase
Guinea Pigs*
Guinea*
Hydrogen
Hydrolysis
Metabolism*
Safflower Oil*
Serine C-Palmitoyltransferase
Sphingomyelin Phosphodiesterase
Ceramidases
Glucosylceramidase
Hydrogen
Safflower Oil
Serine C-Palmitoyltransferase
Sphingomyelin Phosphodiesterase
Figure
Reference
-
1. Elias PM. Epidermal lipids, barrier function, and desquamation. J Invest Dermatol. 1983; 80:1 Suppl. 44s–49s.
Article2. Elias PM, Menon GK. Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv Lipid Res. 1991; 24:1–26.
Article3. Harding CR. The stratum corneum: structure and function in health and disease. Dermatol Ther. 2004; 17:Suppl 1. 6–15.
Article4. Hamanaka S, Hara M, Nishio H, Otsuka F, Suzuki A, Uchida Y. Human epidermal glucosylceramides are major precursors of stratum corneum ceramides. J Invest Dermatol. 2002; 119(2):416–423.
Article5. Uchida Y, Hara M, Nishio H, Sidransky E, Inoue S, Otsuka F, Suzuki A, Elias PM, Holleran WM, Hamanaka S. Epidermal sphingomyelins are precursors for selected stratum corneum ceramides. J Lipid Res. 2000; 41(12):2071–2082.
Article6. Gray GM, Yardley HJ. Lipid compositions of cells isolated from pig, human, and rat epidermis. J Lipid Res. 1975; 16(6):434–440.
Article7. Burr GO, Burr MM. Nutrition classics from The Journal of Biological Chemistry 82:345-67, 1929. A new deficiency disease produced by the rigid exclusion of fat from the diet. Nutr Rev. 1973; 31(8):248–249.8. Wertz PW, Cho ES, Downing DT. Effect of essential fatty acid deficiency on the epidermal sphingolipids of the rat. Biochim Biophys Acta. 1983; 753(3):350–355.
Article9. Prottey C. Essential fatty acids and the skin. Br J Dermatol. 1976; 94(5):579–585.
Article10. Ziboh VA, Chapkin RS. Biologic significance of polyunsaturated fatty acids in the skin. Arch Dermatol. 1987; 123(12):1686a–1690.
Article11. Barre DE. Potential of evening primrose, borage, black currant, and fungal oils in human health. Ann Nutr Metab. 2001; 45(2):47–57.
Article12. Furse RK, Rossetti RG, Zurier RB. Gammalinolenic acid, an unsaturated fatty acid with anti-inflammatory properties, blocks amplification of IL-1 beta production by human monocytes. J Immunol. 2001; 167(1):490–496.13. Chung S, Kong S, Seong K, Cho Y. Gamma-linolenic acid in borage oil reverses epidermal hyperproliferation in guinea pigs. J Nutr. 2002; 132(10):3090–3097.14. Cho Y, Ziboh VA. Nutritional modulation of guinea pig skin hyperproliferation by essential fatty acid deficiency is associated with selective down regulation of protein kinase C-beta. J Nutr. 1995; 125(11):2741–2750.15. Mohrhauer H, Holman RT. The Effect of Dose Level of Essential Fatty Acids Upon Fatty Acid Composition of the Rat Liver. J Lipid Res. 1963; 4:151–159.
Article16. Kim Y, Song EH, Shin K, Lee Y, Cho Y. Dietary effect of silk protein on epidermal levels of free sphingoid bases and phosphate metabolites in NC/Nga mice. Korean J Nutr. 2012; 45(2):113–120.
Article17. Wang LJ, Chen SJ, Chen Z, Cai JT, Si JM. Morphological and pathologic changes of experimental chronic atrophic gastritis (CAG) and the regulating mechanism of protein expression in rats. J Zhejiang Univ Sci B. 2006; 7(8):634–640.
Article18. Takagi Y, Nakagawa H, Yaginuma T, Takema Y, Imokawa G. An accumulation of glucosylceramide in the stratum corneum due to attenuated activity of beta-glucocerebrosidase is associated with the early phase of UVB-induced alteration in cutaneous barrier function. Arch Dermatol Res. 2005; 297(1):18–25.
Article19. Uchida Y, Behne M, Quiec D, Elias PM, Holleran WM. Vitamin C stimulates sphingolipid production and markers of barrier formation in submerged human keratinocyte cultures. J Invest Dermatol. 2001; 117(5):1307–1313.
Article20. Mizutani Y, Mitsutake S, Tsuji K, Kihara A, Igarashi Y. Ceramide biosynthesis in keratinocyte and its role in skin function. Biochimie. 2009; 91(6):784–790.
Article21. Holleran WM, Takagi Y, Uchida Y. Epidermal sphingolipids: metabolism, function, and roles in skin disorders. FEBS Lett. 2006; 580(23):5456–5466.
Article22. Bartke N, Hannun YA. Bioactive sphingolipids: metabolism and function. J Lipid Res. 2009; 50:Suppl. S91–S96.
Article23. Ohnishi Y, Okino N, Ito M, Imayama S. Ceramidase activity in bacterial skin flora as a possible cause of ceramide deficiency in atopic dermatitis. Clin Diagn Lab Immunol. 1999; 6(1):101–104.
Article24. McCullough JL, Schreiber SH, Ziboh VA. Cell proliferation kinetics of epidermis in the essential fatty acid deficient rat. J Invest Dermatol. 1978; 70(6):318–320.
Article25. Tang W, Ziboh VA. Reversal of epidermal hyperproliferation in essential fatty acid deficient guinea pigs is accompanied by rapid generation of inositol triphosphate. Arch Dermatol Res. 1988; 280(5):286–292.
Article26. Kim J, Kim H, Jeong H, Kim SH, Park SK, Cho Y. Comparative effect of gromwell (Lithospermum erythrorhizon) extract and borage oil on reversing epidermal hyperproliferation in guinea pigs. Biosci Biotechnol Biochem. 2006; 70(9):2086–2095.
Article27. Lampe MA, Williams ML, Elias PM. Human epidermal lipids: characterization and modulations during differentiation. J Lipid Res. 1983; 24(2):131–140.
Article28. Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, Lehmann JM. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci U S A. 1997; 94:4318–4323.29. McCusker MM, Grant-Kels JM. Healing fats of the skin: the structural and immunologic roles of the ω-6 and ω-3 fatty acids. Clin Dermatol. 2010; 28(4):440–451.
Article30. HogenEsch H, Boggess D, Sundberg JP. Changes in keratin and filaggrin expression in the skin of chronic proliferative dermatitis (cpdm) mutant mice. Pathobiology. 1999; 67(1):45–50.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Arctii Fructus is a Prominent Dietary Source of Linoleic Acid for Reversing Epidermal Hyperproliferation of Guinea Pigs
- Effects of dietary fish oil on myocardical ischemia and reperfusion in isolated guinea pig heart
- Effect of Dietary Oil Containing gamma-Linolenic Acid on the Plasma Lipid Levels and Thrombotic Activity in Rats
- Evening Primrose (Oenothera biennis) Oil in Management of Female Ailments
- Effects of perilla oil on plasma concentrations of cardioprotective (n-3) fatty acids and lipid profiles in mice