J Korean Soc Endocrinol.
2006 Aug;21(4):281-289. 10.3803/jkes.2006.21.4.281.
Clinical Usefulness of Glucose Testing from the Forearm in Diabetic Patients
- Affiliations
-
- 1Department of Endocrinology and Metabolism, College of Medicine, Korea.
- 2Research Institute of Endocrinology, Kyung Hee University, Korea.
- 3Department of Internal Medicine, Keonyang University, Korea.
- 4Department of Chemistry, Kwangwoon University, Korea.
- KMID: 2200843
- DOI: http://doi.org/10.3803/jkes.2006.21.4.281
Abstract
-
BACKGROUND: Self monitoring of blood glucose plays an important role in the management of diabetes. However, traditional finger prick testing causes pain and so compliance with self monitoring of blood glucose is usually poor. Using an alternative site for sampling may reduce the level of pain and be beneficial for improving the compliance of diabetic patients. We evaluated the accuracy and acceptability of blood glucose testing from the forearm by analyzing the performance of the CareSens(R) (i-Sens, Inc. Korea) device for diabetic patients.
METHODS
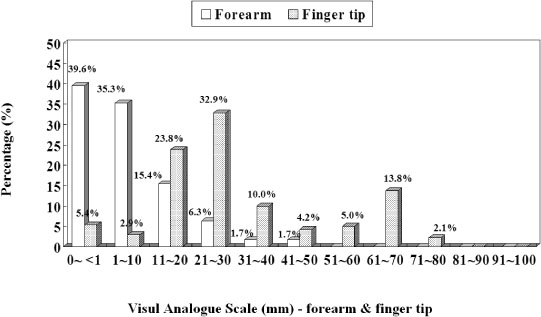
We measured the glucose level at the forearm by use of CareLance(R) (vaccum assisted lancing device) and also at the finger tip simultaneously by use of the CareSens(R) device at fasting and postprandial 2 hours, respectively. At the same time, the glucose levels of venous samples were checked by the laboratory method (BIOSEN 5030, EKF, Germany) and compared with those glucose level measured by the CareSens(R) device. We also checked the ease of use of the CareLance(R) and the associated pain of the patients by means of a visual analogue scale (VAS) at the time of blood sampling.
RESULTS
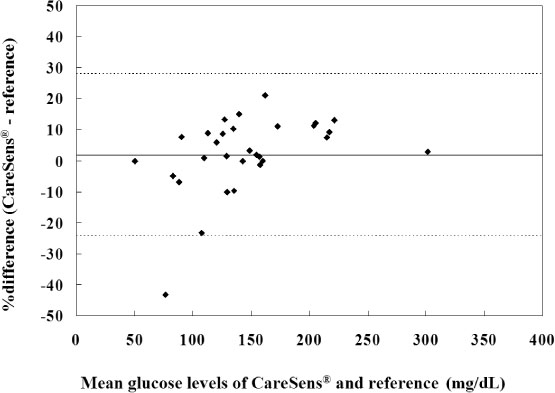
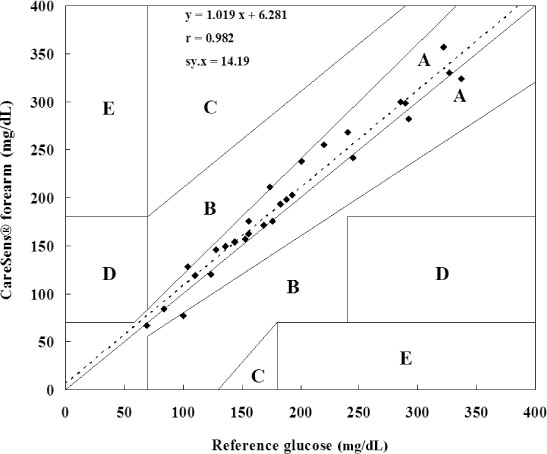
The glucose level obtained from the forearm and finger tip correlated well with that from the laboratory method, respectively. Error grid analysis showed that 100% of the measurements were clinically acceptable; forearm blood glucose testing by use of CareLance(R) was less painful and it was as easy to use as the finger prick (P < 0.05 and P = 0.04, respectively).
CONCLUSION
Forearm testing is an acceptable alternative to finger prick testing for measuring blood glucose in diabetic patients.
MeSH Terms
Figure
Reference
-
1. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998. 352:837–853.2. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in over-weight patients with type 2 diabetes (UKPDS 34). Lancet. 1998. 352:854–865.3. The Diabetes Control and Complications Trial (DCCT) Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993. 329:977–986.4. American Diabetes Association. National standards for Diabetes Self-Management Education programs and American Diabetes Association review criteria. Diabetes Care. 1995. 18:737–741.5. Ary DV, Toobert D, Wilson W, Glasgow RE. Patient perspective on factors contributing to nonadherence to diabetes regimen. Diabetes care. 1986. 9:168–172.6. Evans JM, Newton RW, Ruta DA, MacDonald TM, Stevenson RJ, Morris AD. Frequency of blood glucose monitoring in relation to glycaemic control: observational study with diabetes database. BMJ. 1999. 319:83–86.7. Rhee SY, Kim YS, Oh S, Choi WH, Park JE, Jeong WJ. Diabcare Asia 2001-Korea country report on outcome data and analysis. Korean J Intern Med. 2005. 20:48–54.8. Greenhalgh S, Bradshaw S, Hall CM, Price DA. Forearm blood glucose testing in diabetes mellitus. Arch Dis Child. 2004. 89:516–518.9. Ellison JM, Stegmann JM, Colner SL, Michael RH, Sharma MK, Ervin KR, Horwitz DL. Rapid changes in postprandial blood glucose produce concentration differences at finger, forearm, and thigh sampling sites. Diabetes Care. 2002. 25:961–964.10. Jungheim K, Koschinsky T. Glucose monitoring at the arm: risky delays of hypoglycemia and hyperglycemia detection. Diabetes Care. 2002. 25:956–960.11. Peled N, Wong D, Gwalani SL. Comparison of glucose levels in capillary blood samples obtained from a variety of body sites. Diabetes Technol Ther. 2002. 4:35–44.12. Clarke WL, Cox DJ, Gonder-Frederick LA, Carter W, Pohl SL. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care. 1987. 10:622–628.13. Dedrick RF, Davis WK. What do statistics really tell us about the quality of the data from self-monitoring of blood glucose. Diabet Med. 1989. 6:267–273.14. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986. 327:307–310.15. American Diabetes Association. Self-monitoring of blood glucose. Diabetes Care. 1994. 17:81–86.16. Bina DM, Anderson RL, Johnson ML, Bergenstal RM, Kendall DM. Clinical impact of prandial state, exercise, and site preparation on the equivalence of alternative-site blood glucose testing. Diabetes Care. 2003. 26:981–985.17. McGarraugh G. Response to Jungheim and Koschinsky: Glucose monitoring at the arm (letter). Diabetes Care. 2001. 24:1304–1306.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Depression and Blood Glucose Testing in Women Type2 Diabetic Patients
- Computer Input Frequency of Blood Glucose Self Testing in Type 2 Diabetic Patients
- Usefulness of Dipstick Test for Vitreous Glucose in Autopsy Practice
- Comparison of Blood Glucose Measurements Using Samples Obtained from the Forearm, Finger Skin Puncture, and Venous Serum
- Determinants of the Risk of Diabetic Kidney Disease and Diabetic Retinopathy Independent of Glucose Exposure