J Korean Med Assoc.
2012 Mar;55(3):292-303. 10.5124/jkma.2012.55.3.292.
Proposal for the development of a human biological material management system for research hospitals
- Affiliations
-
- 1Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. mindmader@gmail.com
- 2Department of Medical History and Medical Humanities, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Pathology, Korea University Guro Hospital, Seoul, Korea.
- 4Korea Human Bio-Repositories Network, Seoul, Korea.
- 5Bio-Resource Center, Asan Medical Center, Seoul, Korea.
- KMID: 2192719
- DOI: http://doi.org/10.5124/jkma.2012.55.3.292
Abstract
- In this paper, the authors suggest more effective conditions for human tissue preparation in research hospitals. Because recent genetic and molecular studies have contributed to the rapid development of molecular and genetic medicine, human tissue is now again being considered as a valuable research resource. Basically, high-quality research-oriented tissue bank organizations are a very important part of a research hospital. The current management system for human tissue, however, is not very effective from either legal or practical perspectives. In this article, the authors propose some improvements on the human tissue management system. The laws on human tissue such as the Bioethics and Safety Act should be changed to contain suitable language applying on-site and efficient multi-dimensional information. Informed consent should be an essential requirement before surgery. Pathologists should be supported as the essential manpower of human tissue banks by law. A committee composed of a clinician, researcher, pathologist, information manager, and coordinator should be established to manage human tissue banks in hospitals. The Institutional Review Board should pay more attention to preventing the leakage of the private information of donors, and researchers should know about the review process and guidelines. This suggestion will create a more stable and effective system for the management of human tissue banks, and it will also create a complete and integrated system for research institutions. Therefore, human tissue banks can play an important role in improving research hospitals' competitiveness by providing a valuable collection of material for research.
Keyword
MeSH Terms
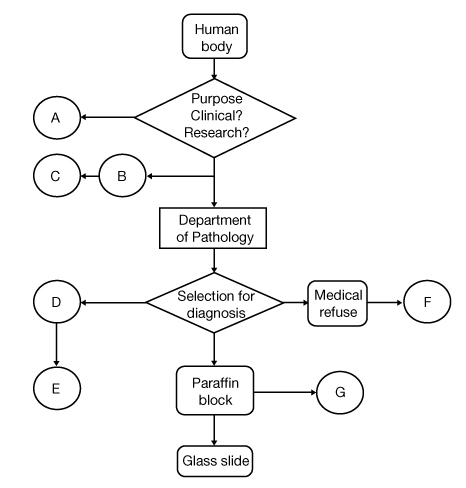
Figure
Cited by 1 articles
-
Let Archived Paraffin Blocks Be Utilized for Research with Waiver of Informed Consent
Yong-Jin Kim, Jeong Sik Park, Karam Ko, Chang Rok Jeong
J Pathol Transl Med. 2018;52(3):141-147. doi: 10.4132/jptm.2018.02.07.
Reference
-
1. Hewitt RE. Biobanking: the foundation of personalized medicine. Curr Opin Oncol. 2011. 23:112–119.
Article2. Giannini C, Oelkers MM, Edwards WD, Aubry MC, Muncil MM, Mohamud KH, Sandleback SG, Nowak JM, Bridgeman A, Brown ME, Cheville JC. Maintaining clinical tissue archives and supporting human research: challenges and solutions. Arch Pathol Lab Med. 2011. 135:347–353.
Article3. Ryu Y, Shin B, Kim B, Kim A, Kim H. Legal and ethical consideration in the use of human biological material. Korean J Pathol. 2010. 44:111–116.
Article4. Bevilacqua G, Bosman F, Dassesse T, Hofler H, Janin A, Langer R, Larsimont D, Morente MM, Riegman P, Schirmacher P, Stanta G, Zatloukal K, Caboux E, Hainaut P. The role of the pathologist in tissue banking: European Consensus Expert Group Report. Virchows Arch. 2010. 456:449–454.
Article5. Hong SY. A critical review on the 'bioethics and biosafety law'. J Korean Bioethics Assoc. 2004. 5:13–23.6. Additional protocol to the convention on human rights and biomedicine, concerning genetic testing for health purposes [Internet]. Council of Europe. 2008. cited 2012 Jan 1. Strasbourg: Council of Europe;Available from: http://conventions.coe.int/Treaty/en/Treaties/html/203.htm.7. Hegtvedt KA. Qualitative studies and the IRB. Social and behavioral research with human subjects: regulations and reasonableness [Internet]. cited 2012 Jan 1. Washington, DC: U.S. Department of Health & Human Services;Available from: http://www.hhs.gov/ohrp/archive/sachrp/mtgings/mtg07-04/present/regreasn_files/frame.htm#slide0008.htm.8. Lee JS, Kim OJ. Regulations and policies on research misconduct: with special reference to the Office of Research Integrity in the United States. J Korean Bioethics Assoc. 2006. 7:101–116.9. 42 CFR Parts 50 and 93: public health service policies on research misconduct [Internet]. 2005. cited 2011 Aug 1. Rockville: U.S. Department of Health and Human Services;Available from: http://ori.dhhs.gov/documents/FR_Doc_05-9643.shtml.10. Kozlowski LT, Vogler GP, Vandenbergh DJ, Strasser AA, O'Connor RJ, Yost BA. Using a telephone survey to acquire genetic and behavioral data related to cigarette smoking in "made-anonymous" and "registry" samples. Am J Epidemiol. 2002. 156:68–77.
Article11. The report of the Royal Liverpool Children's Inquiry [Internet]. The Royal Liverpool Children's Inquiry. 2001. cited 2012 Jan 1. Norwich: The Royal Liverpool Children's Inquiry;Available from: http://www.rlcinquiry.org.uk.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of B2B E-Commerce in the Material Management of Hospitals
- Legal and Ethical Consideration in the Use of Human Biological Material
- Factors Influencing on Operation Efficiency of Information Management System for Supply and Demand of Materials at Health Care: Case Study in General Hospitals of Daejeon City
- Financial Projection of the Nursing Fee Differentiation Policy Improvement Proposal in the National Health Insurance: Using a Break-even Analysis Model for the Optimal Nursing Fee
- Integrated Pharmaceutical Supply Chain Management based on B2B Collaboration and Information Sharing