J Korean Med Assoc.
2008 Sep;51(9):838-848. 10.5124/jkma.2008.51.9.838.
Imaging Findings of Missed Breast Cancer: Retrospective Analysis
- Affiliations
-
- 1Department of Radiolgy, Sungkyunkwan University School of Medicine, Korea. bkhan@skku.edu
- KMID: 2185963
- DOI: http://doi.org/10.5124/jkma.2008.51.9.838
Abstract
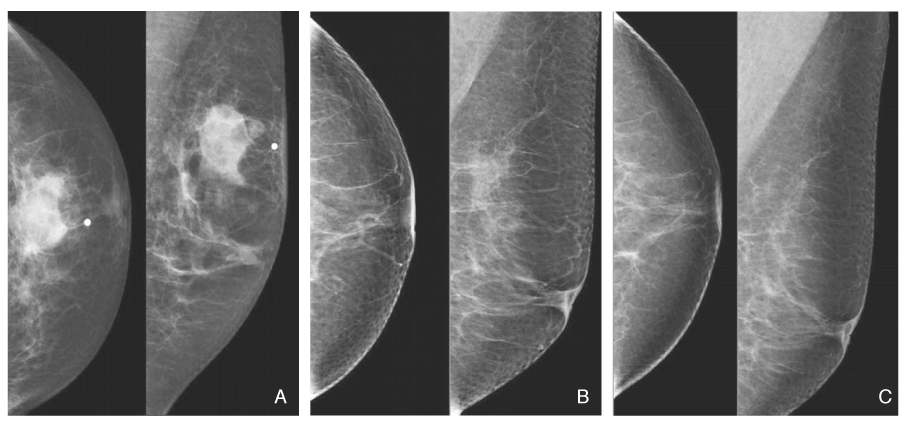
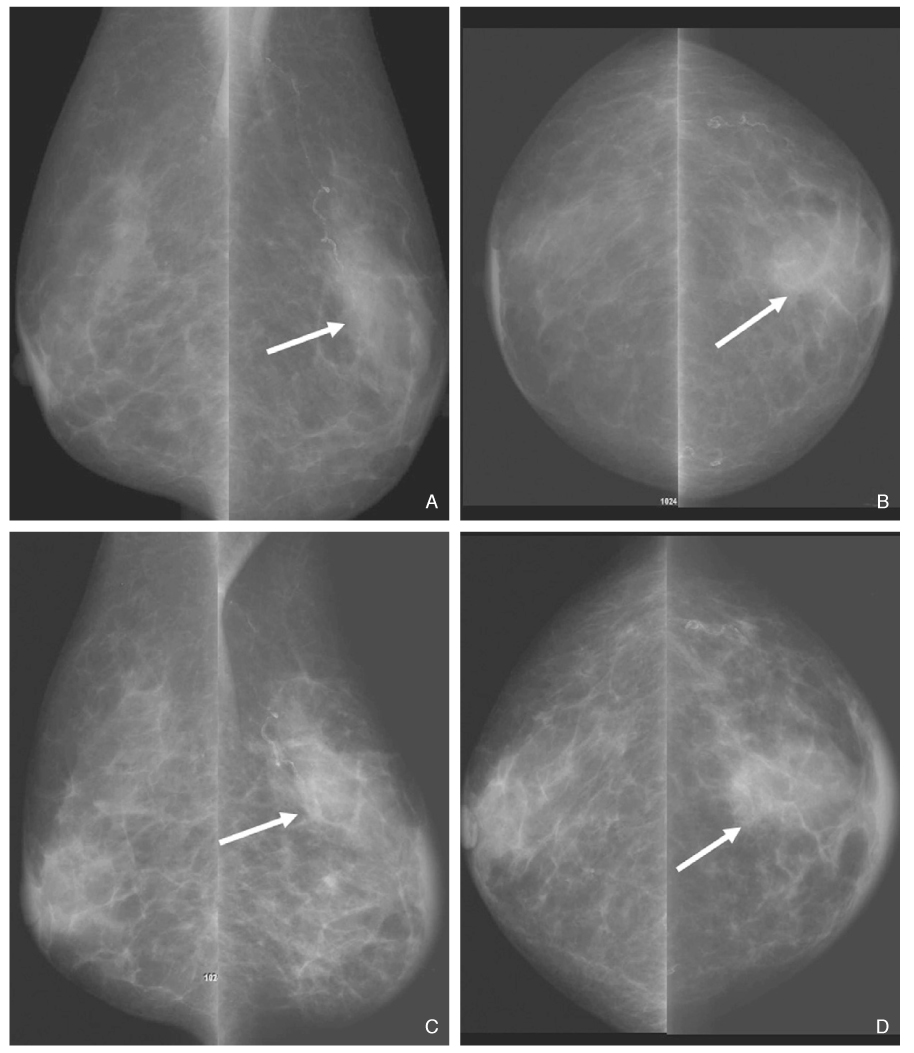
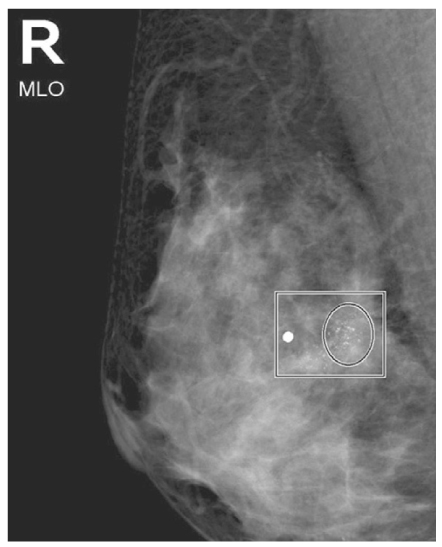
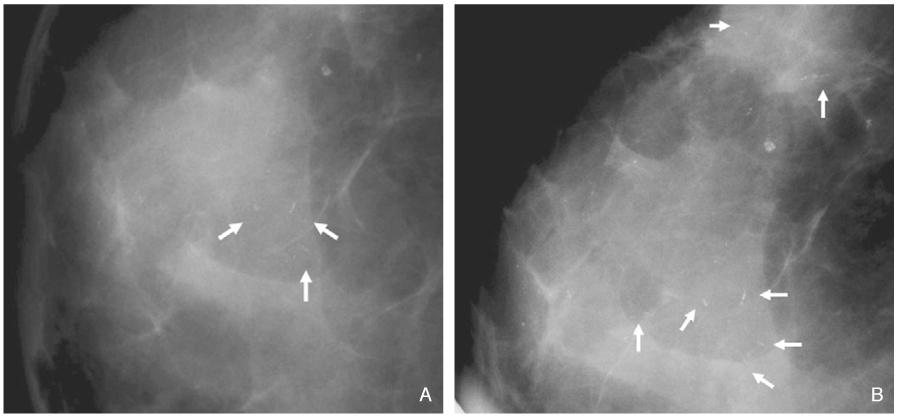
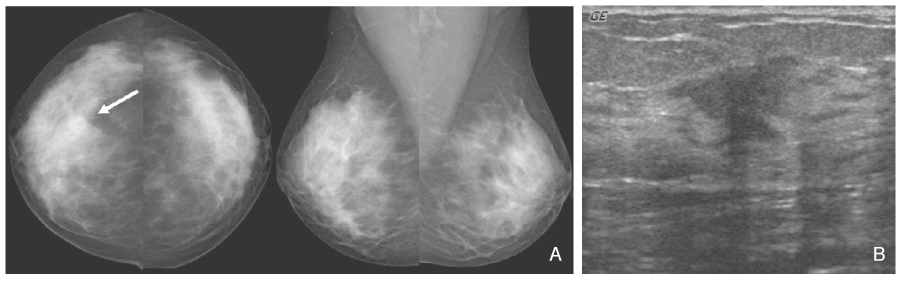
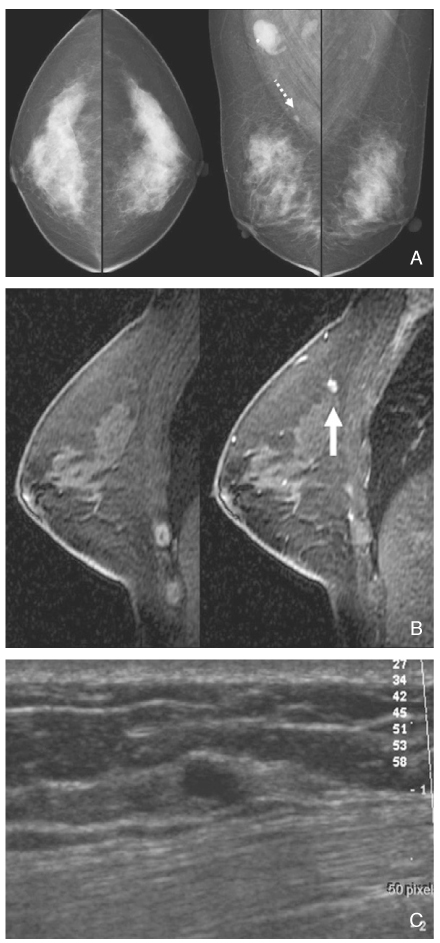
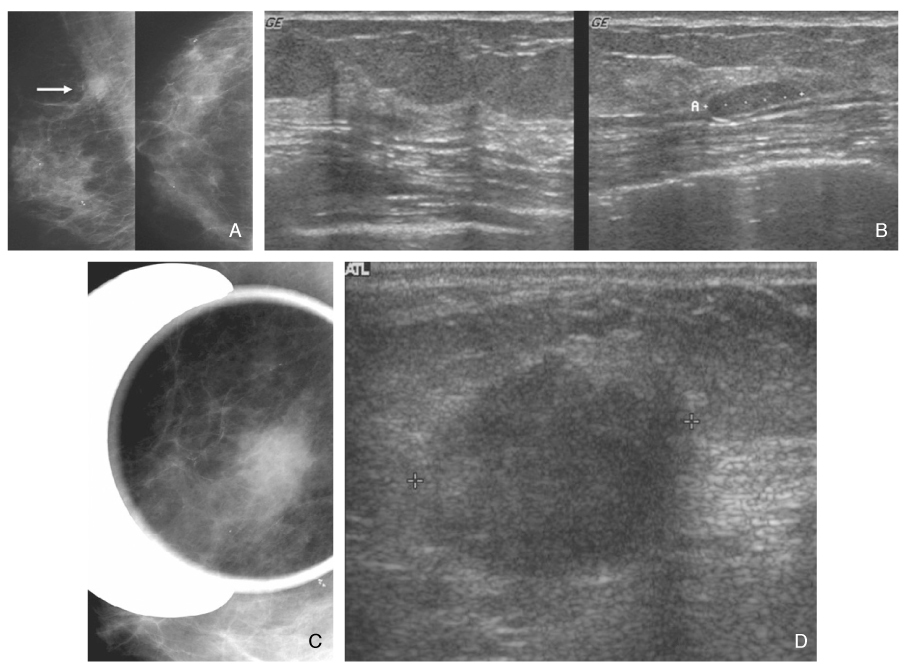
- Screening mammography has been proved to be an effective tool to detect early breast cancers and to decrease mortality. However, the rate of false-negative mammograms has been reported to be still high as 10~30%. Missed breast cancers are cancers that are visible at previous mammograms only retrospectively and can be classified as three types; interval cancers, subsequent screen-detected cancers, and alternative imaging-detected cancers. In a small group, screen-detected abnormalities recalled for further evaluation may be dismissed due to false negative diagnostic assessment, leading to delays in breast cancer diagnosis. Possible causes for missing include perception errors, interpretation errors, and technical errors. Furthermore, every diagnostic examination has inherent limitations. Perception errors are often attributed to combined multiple factors; peripheral lesions, single view abnormalities, subtle findings, distracting lesions, and dense parenchyma obscuring a lesion. To decrease the false negative rate, radiologists should be alert to take additional mammograms and ultrasonography, and should try to improve the image quality and interpretation techniques comparing with the previous imaging, considering the use of computer-aided detection or double reading.
Keyword
MeSH Terms
Figure
Reference
-
1. Fletcher SW, Black W, Harris RP, Rimer BK, Shapiro S. Report of the International Workshop on Screening for Breast Cancer. J Natl Cancer Inst. 1993. 85:1644–1656.
Article2. Harris R, Leininger L. Clinical strategies for breast cancer screening: weighing and using the evidence. Ann Intern Med. 1995. 112:539–547.
Article3. Kerlikowske K, Grady D, Rubin S, Sandrock C, Ernster VL. Efficacy of screening mammography. A meta-analysis. JAMA. 1995. 273:149–154.
Article4. Tabár L, Fagerberg CJ, Gad A, Baldetorp L, Holmberg LH, Gröntoft O, Ljungquist U, Lundström B, Månson JC, Eklund G. Reduction in mortality from breast cancer after mass screening with mammography. Randomised trial from the Breast Cancer Screening Working Group of the Swedish National Board of Health and Welfare. Lancet. 1985. 1:829–832.5. Houssami N, Irwig L, Ciatto S. Radiological surveillance of interval breast cancers in screening programmes. Lancet Oncol. 2006. 7:259–265.
Article6. Warren RM, Young JR, McLean L, Lyons K, Wilson AR, Evans A, Duffy SW, Warsi IM. Radiology review of the UKCCCR Breast Screening Frequency Trial: potential improvements in sensitivity and lead time of radiological signs. Clin Radiol. 2003. 58:128–132.
Article7. Harvey JA, Fajardo LL, Innis CA. Preview mammograms on patients with impalpable breast carcinoma: Retrospective vs blind interpretation. AJR Am J Roentgenol. 1993. 161:1167–1172.
Article8. Warren R, Duffy S. Interval cancers as an indicator of performance in breast screening. Breast Cancer. 2000. 7:9–18.
Article9. Buist DS, Porter PL, Lehnman C, Taplin SH, White E. Factors contributing to mammography failure in women aged 40-49 years. J Natl Cancer Inst. 2004. 96:1432–1440.
Article10. Brenner RJ. False-negative mammograms. Medical, legal, and risk management implications. Radiol Clin North Am. 2000. 38:741–757.11. Han BK, Kim JY, Shin JH, Choi YH. Performance of computer-aided detection in false negative screening mammograms of breast cancers. J Korean Radiol Soc. 2004. 51:465–472.
Article12. Soo MS, Baker JA, Rosen EL. Sonographic detection and sonographically guided biopsy of breast microcalcifications. AJR Am J Roentgenol. 2003. 180:941–948.
Article13. Majid AS, de Paredes ES, Doherty RD, Sharma NR, Salvador X. Missed Breast Carcinoma: Pitfalls and Pearls. Radiographics. 2003. 23:881–895.
Article