J Korean Med Assoc.
2007 Sep;50(9):807-814. 10.5124/jkma.2007.50.9.807.
Research on Uterine Cervical Cancer in Korea: Current Status and Perspectives
- Affiliations
-
- 1Department of Obstetrics and Gynecology, University of Ulsan College of Medicine, Korea. jhnam@amc.seoul.kr
- KMID: 2184917
- DOI: http://doi.org/10.5124/jkma.2007.50.9.807
Abstract
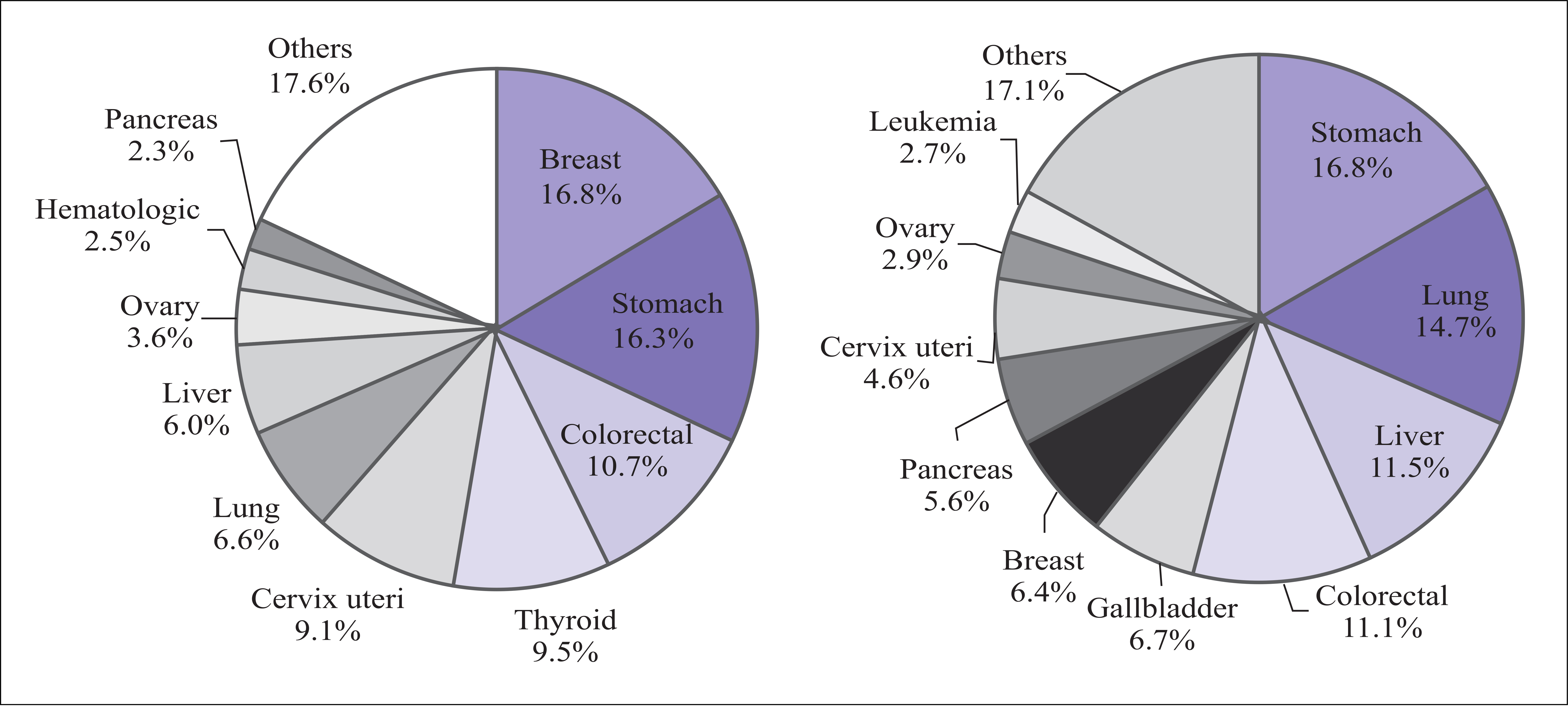
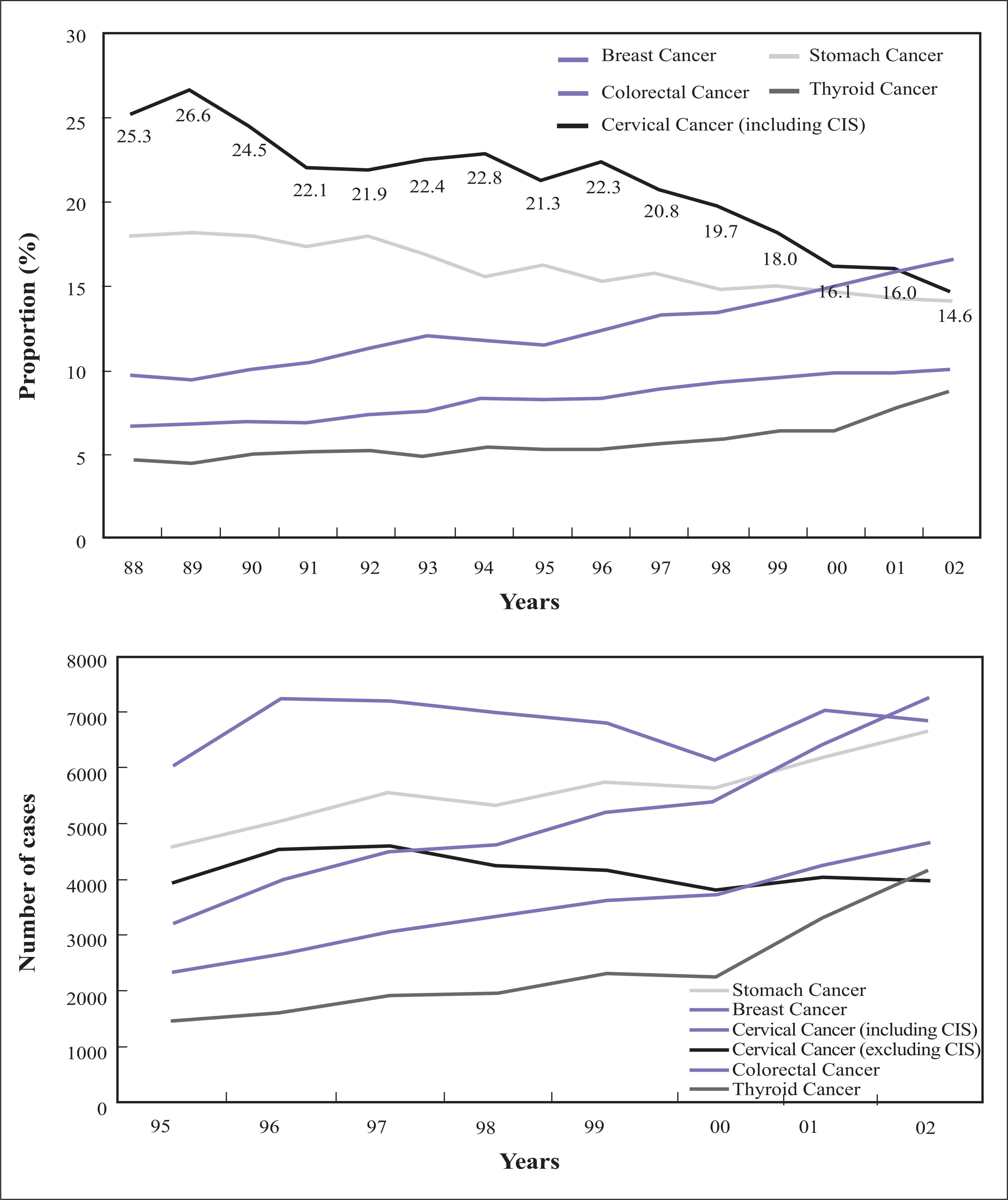
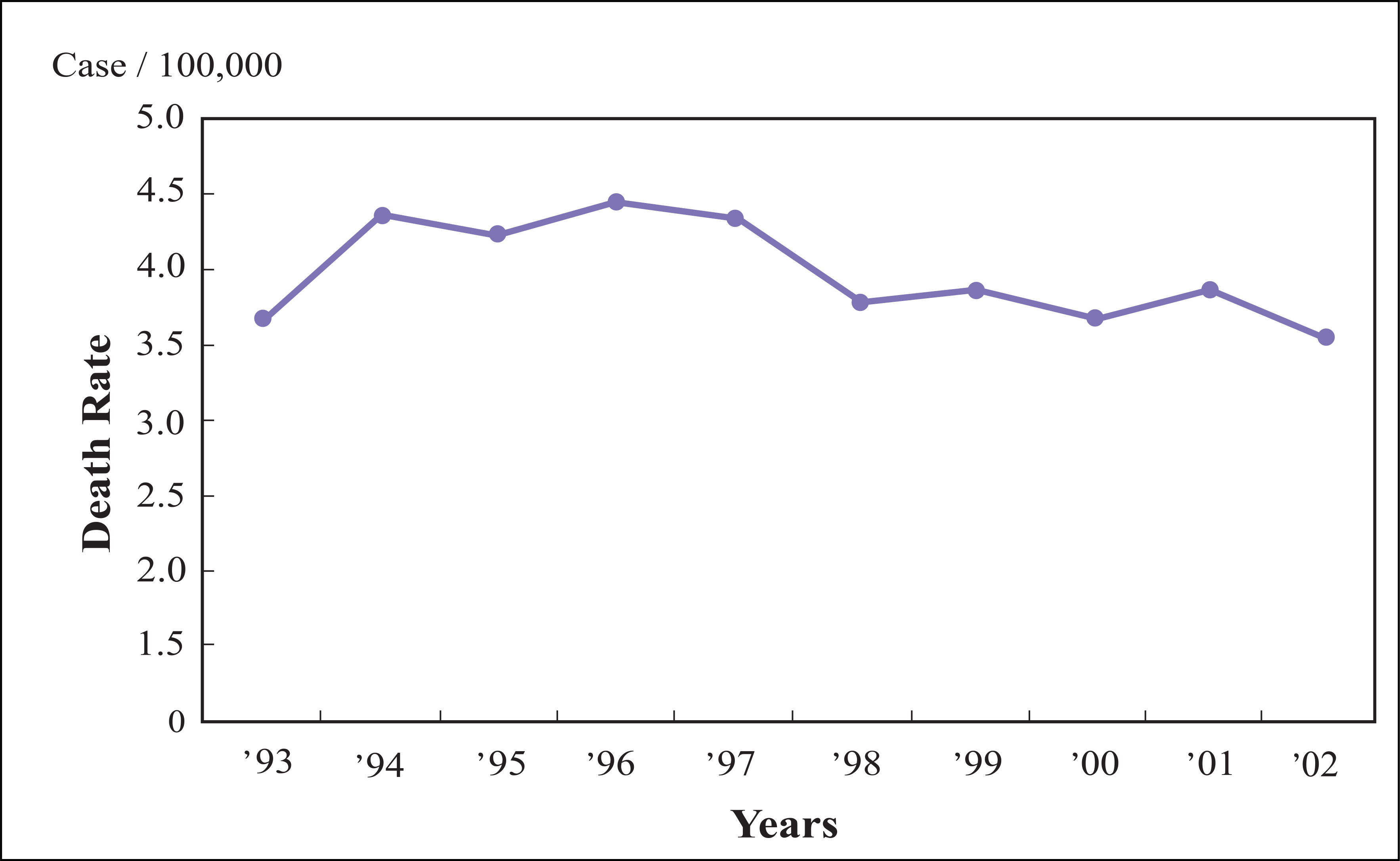
- In Korea, cervical cancer is the fifth most common female cancer and the eighth most common cause of female cancer deaths. It is the most frequently diagnosed gynecologic cancer. Over the past decades, a marked reduction in the incidence and mortality of this malignancy has been documented. This has been largely attributed to widespread screening using papanicolaou smear, the early detection and treatment of preivasive lesions, and development of an effective treatment modality for invasive cervical cancer. The recently developed human papilomavirus vaccine is expected to further reduce the incidence and mortality of this malignancy markedly. Epidemiologic, biologic, and clinical research on cervical cancer is carried out worldwide to introduce human papillomavirus test and vaccine and to develop effective treatment for invasive cancer. At this point of time, the author reviewed the present status of research on cervical cancer and proposed future studies required to reduce the incidence and mortality of this malignancy in Korea.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Minstry of Health and Welfare. 2002 Annual report of Korea Central Cancer Registry. Seoul. 2003.2. Korean Society of Obstetrics and Gynecology. Annual Report of Gynecologic Cancer Registry Program in Korea for 2004 (Jan. 1st, 2004?Dec. 31st, 2004). Korean J Obstet Gynecol. 2006; 49:762–812.3. Chung HH, Jang MJ, Jung KW, Won YJ, Shin HR, Kim JW, Lee HP. Cervical cancer incidence and survival in Korea: 1993?2002. Int J Gynecol Cancer. 2006; 16:1833–1838.
Article4. Devesa SS, Young JL, Brinton LA, Fraumeni JF. Recent trends in cervix uteri cancer. Cancer. 1989; 64:2184–2190.
Article5. Eide TJ. Cancer of the uterine cervix in Norway by histologic type, 1970–1984. J Natl Cancer Inst. 1987; 79:199–205.6. Nieminen P, Kallio M, Hakama M. The effect of mass screening on incidence and mortality of squamous and adenocarcinoma of cervix uteri. Obstet Gynecol. 1995; 85:1017–1021.
Article7. Khang SK, Park SY. Guidelines for the screening of uterine cervical cancer. J Korean Med Assoc. 2002; 45:1005–14.
Article8. Miller AB, Anderson G, Brisson J, Laidlaw J, Le Pitre N, Malcolmson P, Mirwaldt P, Stuart G, Sullivan W. Report of a National Workshop on Screening for Cancer of the Cervix. CMAJ. 1991; 145:1301–1325.9. Sato S, Makino H, Yajima A, Fukao A. Cervical cancer screening in Japan. A case?control study. Acta Cytol. 1997; 41:1103–1106.10. Patnick J. Cervical cancer screening in England. Eur J Cancer. 2000; 36:2205–2208.
Article11. Schaffer P, Sancho?Garnier H, Fender M, Dellenbach P, Carbillet JP, Monnet E, Gauthier GP, Garnier A. Cervical cancer screening in France. Eur J Cancer. 2000; 36:2215–2220.
Article12. Schenck U, von Karsa L. Cervical cancer screening in Germany. Eur J Cancer. 2000; 36:2221–2226.
Article13. Segnan N, Ronco G, Ciatto S. Cervical cancer screening in Italy. Eur J Cancer. 2000; 36:2235–2239.
Article14. Nanda K, McCrory DC, Myers ER, Bastian LA, Hasselblad V, Hickey JD, Matchar DB. Accuracy of the Papanicolaou test in screening for and follow?up of cervical cytologic abnormalities: a systematic review. Ann Intern Med. 2000; 132:810–819.
Article15. Song MK, Kwon YI, Park TC, Chon MJ, Shin JW, Lee JW, Lee JM, Namkoong SE. False negative cytology in cervical smears: An evaluation of 186 cases of squamous intraepithelial lesion and squamous cell carcinoma, hitologically confirmed. Korean J Obstet Gynecol. 2001; 44:763–768.16. Wright TC, Denny L, Kuhn L, Pollack A, Lorincz A. HPV DNA testing of self?collected vaginal samples compared with cytologic screening to detect cervical cancer. JAMA. 2000; 283:81–86.
Article17. Schiffman M, Herrero R, Hildesheim A, Sherman ME, Bratti M, Wacholder S, Alfaro M, Hutchinson M, Morales J, Greenberg MD, Lorincz AT. HPV DNA testing in cervical cancer screening: results from women in a high?risk province of Costa Rica. JAMA. 2000; 283:87–93.
Article18. Kulasingam SL, Hughes JP, Kiviat NB, Mao C, Weiss NS, Kuypers JM, Koutsky LA. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA. 2002; 288:1749–1757.19. Bollen LJ, Tjong AHSP, van der Velden J, Mol BW, Lammes FB, ten Kate FW, ter Schegget J, Bleker OP. Human papillomavirus DNA after treatment of cervical dysplasia: low prevalence in normal cytologic smears. Cancer. 1996; 77:2538–2543.
Article20. Elfgren K, Bistoletti P, Dillner L, Walboomers JM, Meijer CJ, Dillner J. Conization for cervical intraepithelial neoplasia is followed by disappearance of human papillomavirus deoxyribonucleic acid and a decline in serum and cervical mucus antibodies against human papillomavirus antigens. Am J Obstet Gynecol. 1996; 174:937–942.
Article21. Solomon D, Schiffman M, Tarone R. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst. 2001; 93:293–299.
Article22. Manos MM, Kinney WK, Hurley LB, Sherman ME, Shieh? Ngai J, Kurman RJ, Ransley JE, Fetterman BJ, Hartinger JS, McIntosh KM, Pawlick GF, Hiatt RA. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA. 1999; 281:1605–1610.23. Kim JJ, Wright TC, Goldie SJ. Cost?effectiveness of human papillomavirus DNA testing in the United Kingdom, The Netherlands, France, and Italy. J Natl Cancer Inst. 2005; 97:888–895.
Article24. Goldie SJ, Gaffikin L, Goldhaber?Fiebert JD, Gordillo?Tobar A, Levin C, Mahe C, Wright TC. Cost?effectiveness of cervical? cancer screening in five developing countries. N Engl J Med. 2005; 353:2158–2168.25. Shin HR, Lee DH, Herrero R, Smith JS, Vaccarella S, Hong SH, Jung KY, Kim HH, Park UD, Cha HS, Park S, Touze A, Munoz N, Snijders PJ, Meijer CJ, Coursaget P, Franceschi S. Prevalence of human papillomavirus infection in women in Busan, South Korea. Int J Cancer. 2003; 103:413–421.
Article26. Shin HR, Franceschi S, Vaccarella S, Roh JW, Ju YH, Oh JK, Kong HJ, Rha SH, Jung SI, Kim JI, Jung KY, van Doorn LJ, Quint W. Prevalence and determinants of genital infection with papillomavirus, in female and male university students in Busan, South Korea. J Infect Dis. 2004; 190:468–476.
Article27. Bosch FX. The Etiology of Squamous Cell Cervical Cancer. In: Rohan TE, Shah KV, ed. Cervical Cancer: From Etiology to Prevention. Dordrecht: Kluwer Academic Publishers. 2006. 191–216.28. zur Hausen H. Human papillomaviruses and their possible role in squamous cell carcinomas. Curr Top Microbiol Immunol. 1977; 78:1–30.
Article29. IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans. Human Papillomaviruses. Lyon. 1995. 64.30. Villa LL, Costa RL, Petta CA, Andrade RP, Paavonen J, Iversen OE, Olsson SE, Hoye J, Steinwall M, Riis?Johannessen G, Andersson?Ellstrom A, Elfgren K, Krogh G, Lehtinen M, Malm C, Tamms GM, Giacoletti K, Lupinacci L, Railkar R, Taddeo FJ, Bryan J, Esser MT, Sings HL, Saah AJ, Barr E. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus?like particle vaccine through 5 years of follow?up. Br J Cancer. 2006; 95:1459–1466.
Article31. Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli?Martins CM, Jenkins D, Schuind A, Costa Clemens SA, Dubin G. Sustained efficacy up to 4.5 years of a bivalent L1 virus?like particle vaccine against human papillomavirus types 16 and 18: follow?up from a randomised control trial. Lancet. 2006; 367:1247–1255.
Article32. Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, Favini G, Ferri L, Mangioni C. Randomised study of radical surgery versus radiotherapy for stage Ib?IIa cervical cancer. Lancet. 1997; 350:535–540.
Article33. Delgado G, Bundy BN, Fowler WC, Stehman FB, Sevin B, Creasman WT, Major F, DiSaia P, Zaino R. A prospective surgical pathological study of stage I squamous carcinoma of the cervix: a Gynecologic Oncology Group Study. Gynecol Oncol. 1989; 35:314–320.
Article34. Nam JH, Kim JH, Kim DY, Kim MK, Yoo HJ, Kim YM, Kim YT, Mok JE. Comparative study of laparoscopico?vaginal radical hysterectomy and abdominal radical hysterectomy in patients with early cervical cancer. Gynecol Oncol. 2004; 92:277–283.
Article35. Ryu HS, Kang SB, Kim KT, Chang KH, Kim JW, Kim JH. Efficacy of different types of treatment in FIGO stage IB2 cervical cancer in Korea: results of a multicenter retrospective Korean study(KGOG?1005). Int J Gynecol Cancer. 2007; 17:132–136.36. NCI Clinical Announcement, US Dept. of Health and Human Services, Publinc Health Service, National Institutes of Health, February. 1999.37. Chung HH, Lee S, Sim JS, Kim JY, Seo SS, Park SY, Roh JW. Pretreatment laparoscopic surgical staging in locally advanced cervical cancer: preliminary results in Korea. Gynecol Oncol. 2005; 97:468–475.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Status of and Perspectives on Cervical Cancer Screening in Korea
- Current status of human papillomavirus vaccines
- Current and Next-generation Vaccines against Human Papillomaviruses
- A Case of Breast Cancer Metastatic Solely to the Uterine Cervix
- Magnivisualizer in the early detection of cervical neoplasia