J Clin Neurol.
2015 Oct;11(4):372-375. 10.3988/jcn.2015.11.4.372.
Pathologic Finding of Thymic Carcinoma Accompanied by Myasthenia Gravis
- Affiliations
-
- 1Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- 2Department of Neurology, National Medical Center, Seoul, Korea.
- 3Department of Neurology, Hallym University College of Medicine, Kangnam Sacred Heart Hospital, Seoul, Korea. yangki2@unitel.co.kr
- KMID: 2179757
- DOI: http://doi.org/10.3988/jcn.2015.11.4.372
Abstract
- BACKGROUND AND PURPOSE
The World Health Organization (WHO) has classified thymic carcinoma and other thymomas (types A, AB, and B) as different neoplasms. Myasthenia gravis (MG) is an early sign of thymoma and theoretically does not accompany thymic carcinoma; however, cases of thymic carcinoma with MG have been reported. Whether thymic carcinoma can accompany MG has yet to be established.
METHODS
The medical records of patients who underwent thymectomy for MG between 1990 and 2011 in a single hospital were reviewed. All cases with the diagnostic code of "thymic carcinoma" or "thymoma type C" (old terminology) were selected. A pathologist re-reviewed the pathologic specimens using the new WHO criteria. The rate of thymic carcinoma among these MG patients was then calculated.
RESULTS
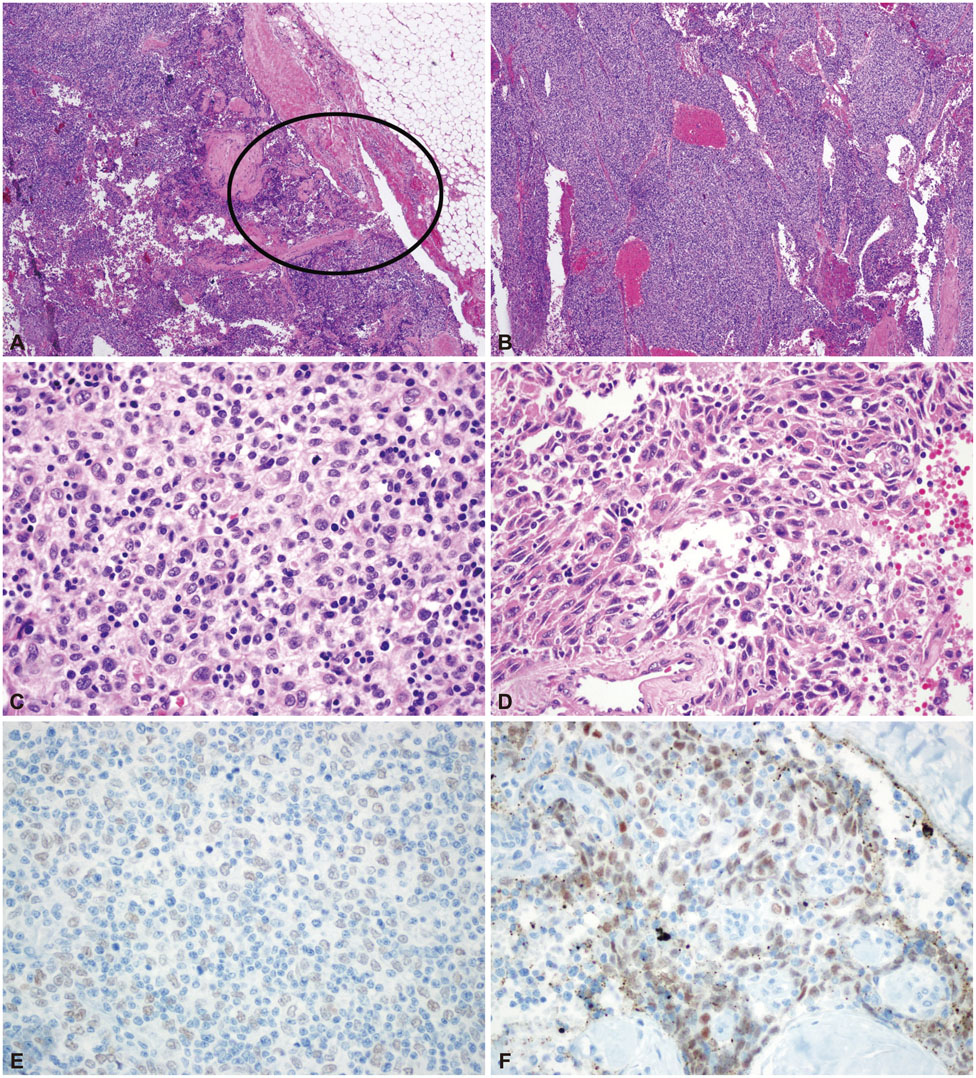
A total of 81 patients with MG had thymic tumors, 10 of whom had thymic carcinomas or thymoma type C. Seven cases of well-differentiated thymic carcinomas (type B3) were excluded, leaving three (3.7%) cases of thymic carcinoma with MG. All three of these cases were type B3 thymoma with a focal squamous cell carcinoma component that was very small and well demarcated. In addition, two out of the three tumors were found to be at an early clinical stage. All of the cases survived without recurrence over follow-up periods of at least 5 years.
CONCLUSIONS
Thymic carcinoma transformation from thymoma can occur during the early stages of thymoma. The association of this condition with MG is not as rare as was previously thought. Thymic carcinomas accompanying MG had a predominant B3 thymoma component with a focal thymic carcinoma area (squamous cell carcinoma).
MeSH Terms
Figure
Reference
-
1. Marulli G, Schiavon M, Perissinotto E, Bugana A, Di Chiara F, Rebusso A, et al. Surgical and neurologic outcomes after robotic thymectomy in 100 consecutive patients with myasthenia gravis. J Thorac Cardiovasc Surg. 2013; 145:730–735. discussion 735-736
Article2. Goldstein SD, Yang SC. Assessment of robotic thymectomy using the Myasthenia Gravis Foundation of America Guidelines. Ann Thorac Surg. 2010; 89:1080–1085. discussion 1085-1086
Article3. Seong YW, Kang CH, Choi JW, Kim HS, Jeon JH, Park IK, et al. Early clinical outcomes of robot-assisted surgery for anterior mediastinal mass: its superiority over a conventional sternotomy approach evaluated by propensity score matching. Eur J Cardiothorac Surg. 2014; 45:e68–e73. discussion e73
Article4. Ettinger DS, Riely GJ, Akerley W, Borghaei H, Chang AC, Cheney RT, et al. Thymomas and thymic carcinomas: Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2013; 11:562–576.5. Müller-Hermelink HK, Engel P, Kuo TT, Ströbel P, Marx A, Harris NL, et al. Tumours of the thymus: introduction. In : Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC, editors. Pathology and genetics: tumours of the lung, pleura, thymus, and heart. Lyon: IARC Press;2004. p. 148–151.6. Marx A, Willcox N, Leite MI, Chuang WY, Schalke B, Nix W, et al. Thymoma and paraneoplastic myasthenia gravis. Autoimmunity. 2010; 43:413–427.
Article7. Yu L, Zhang XJ, Ma S, Jing Y, Li F, Krasna MJ. Different characteristics of thymomas with and without myasthenia gravis. Ann Surg Oncol. 2012; 19:94–98.
Article8. Marx A, Ströbel P, Zettl A, Chan JK, Müller-Hermelink HK, Harris NL, et al. Thymomas. In : Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC, editors. Pathology and genetics: tumours of the lung, pleura, thymus, and heart. . Lyon: IARC Press;2004. p. 152–153.9. Ruffini E, Filosso PL, Mossetti C, Bruna MC, Novero D, Lista P, et al. Thymoma: inter-relationships among World Health Organization histology, Masaoka staging and myasthenia gravis and their independent prognostic significance: a single-centre experience. Eur J Cardiothorac Surg. 2011; 40:146–153.
Article10. Nakagawa K, Asamura H, Matsuno Y, Suzuki K, Kondo H, Maeshima A, et al. Thymoma: a clinicopathologic study based on the new World Health Organization classification. J Thorac Cardiovasc Surg. 2003; 126:1134–1140.
Article11. Kuo TT, Chan JK. Thymic carcinoma arising in thymoma is associated with alterations in immunohistochemical profile. Am J Surg Pathol. 1998; 22:1474–1481.
Article12. Suster S, Moran CA. Primary thymic epithelial neoplasms showing combined features of thymoma and thymic carcinoma. A clinicopathologic study of 22 cases. Am J Surg Pathol. 1996; 20:1469–1480.
Article13. Moran CA, Weissferdt A, Kalhor N, Solis LM, Behrens C, Wistuba II, et al. Thymomas I: a clinicopathologic correlation of 250 cases with emphasis on the World Health Organization schema. Am J Clin Pathol. 2012; 137:444–450.14. Kirchner T, Schalke B, Buchwald J, Ritter M, Marx A, Müller-Hermelink HK. Well-differentiated thymic carcinoma. An organotypical low-grade carcinoma with relationship to cortical thymoma. Am J Surg Pathol. 1992; 16:1153–1169.15. Ströbel P, Marx A, Zettl A, Müller-Hermelink HK. Thymoma and thymic carcinoma: an update of the WHO Classification 2004. Surg Today. 2005; 35:805–811.
Article16. Zettl A, Ströbel P, Wagner K, Katzenberger T, Ott G, Rosenwald A, et al. Recurrent genetic aberrations in thymoma and thymic carcinoma. Am J Pathol. 2000; 157:257–266.
Article17. Ströbel P, Hohenberger P, Marx A. Thymoma and thymic carcinoma: molecular pathology and targeted therapy. J Thorac Oncol. 2010; 5:10 Suppl 4. S286–S290.
Article18. Badve S, Goswami C, Gökmen-Polar Y, Nelson RP Jr, Henley J, Miller N, et al. Molecular analysis of thymoma. PLoS One. 2012; 7:e42669.
Article19. Syrios J, Diamantis N, Fergadis E, Katsaros L, Logothetis M, Iakovidou I, et al. Advances in thymic carcinoma diagnosis and treatment: a review of literature. Med Oncol. 2014; 31:44.
Article20. Ye B, Tantai JC, Ge XX, Li W, Feng J, Cheng M, et al. Surgical techniques for early-stage thymoma: video-assisted thoracoscopic thymectomy versus transsternal thymectomy. J Thorac Cardiovasc Surg. 2014; 147:1599–1603.
Article