J Clin Neurol.
2011 Jun;7(2):69-76. 10.3988/jcn.2011.7.2.69.
Plaque Rupture is a Determinant of Vascular Events in Carotid Artery Atherosclerotic Disease: Involvement of Matrix Metalloproteinases 2 and 9
- Affiliations
-
- 1Department of Neurology, Kyung Hee University School of Medicine, Seoul, Korea. hsyoon96@medimail.co.kr
- 2Department of Biochemistry and Molecular Biology, Kyung Hee University School of Medicine, Seoul, Korea. sky9999@khu.ac.kr
- 3Department of Pathology, Kyung Hee University School of Medicine, Seoul, Korea.
- 4Department of Thoracic and Cardiovascular Surgery, Kyung Hee University School of Medicine, Seoul, Korea.
- KMID: 2178969
- DOI: http://doi.org/10.3988/jcn.2011.7.2.69
Abstract
- BACKGROUND AND PURPOSE
Unstable carotid atherosclerotic plaques are characterized by cap rupture, leading to thromboembolism and stroke. Matrix metalloproteinases (MMPs) have been implicated in the progression of atherosclerosis and plaque rupture. The aim of this study was to assess the relationship between the expressions of MMP-2 and MMP-9 and carotid plaque instability.
METHODS
Eighty atherosclerotic plaques were collected from 74 patients undergoing carotid endarterectomy. Clinical information was obtained from each patient, and plaque morphology was examined at the macroscopic and microscopic levels. The immunohistochemical expressions of MMPs were graded using semiquantitative scales.
RESULTS
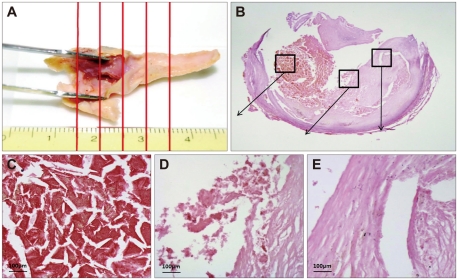
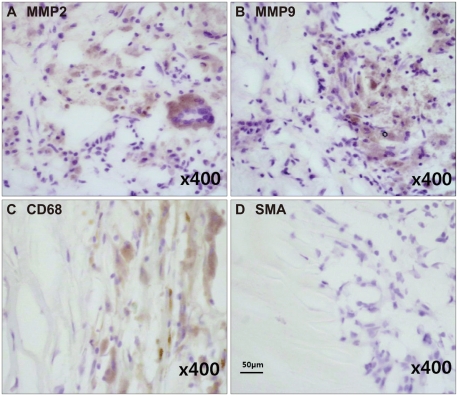
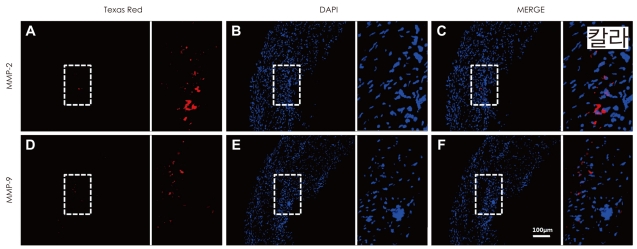
Macroscopic ulceration (84.6% versus 63.4%, p=0.042) and microscopic cap rupture (79.5% versus 51.2%, p=0.010) were more common in symptomatic than in asymptomatic patients. Immunoreactivities of MMP-2 and MMP-9 were increased in 40 and 36 atheromatous plaques, respectively. Macroscopic ulceration was strongly correlated with the expressions of MMP-2 (p<0.001) and MMP-9 (p=0.001). There were significant correlations between increased MMP-2 expression and cap rupture (p=0.002), intraplaque hemorrhage (p=0.039), and a thin fibrous cap (p=0.002), and between increased MMP-9 expression and cap rupture (p=0.010) and a large lipid core (p=0.013).
CONCLUSIONS
Plaque rupture was significantly associated with the development of vascular events in carotid atherosclerotic disease. MMP-2 and MMP-9 are strongly correlated with plaque instability.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Preoperative Coronary Stenosis Is a Determinant of Early Vascular Outcome after Carotid Endarterectomy
Jung Hwa Kim, Sung Hyuk Heo, Hyo Jung Nam, Hyo Chul Youn, Eui-Jong Kim, Ji Sung Lee, Young Seo Kim, Hyun Young Kim, Seong-Ho Koh, Dae-Il Chang
J Clin Neurol. 2015;11(4):364-371. doi: 10.3988/jcn.2015.11.4.364.Multimarker Approach in Discriminating Patients with Symptomatic and Asymptomatic Atherosclerotic Carotid Artery Stenosis
Piotr Musialek, Wieslawa Tracz, Lukasz Tekieli, Piotr Pieniazek, Anna Kablak-Ziembicka, Tadeusz Przewlocki, Ewa Stepien, Przemyslaw Kapusta, Rafal Motyl, Jakub Stepniewski, Anetta Undas, Piotr Podolec
J Clin Neurol. 2013;9(3):165-175. doi: 10.3988/jcn.2013.9.3.165.
Reference
-
1. Hachinski V. Stroke in Korean. Stroke. 2008; 39:1067. PMID: 18323471.2. Katsumata T, Nishiyama Y, Yamaguchi H, Otori T, Nakamura H, Tanaka N, et al. Extracranial carotid plaque is increasing in Japanese ischemic stroke patients. Acta Neurol Scand. 2007; 116:20–25. PMID: 17587251.
Article3. Nagao T, Sadoshima S, Ibayashi S, Takeya Y, Fujishima M. Increase in extracranial atherosclerotic carotid lesions in patients with brain ischemia in Japan. An angiographic study. Stroke. 1994; 25:766–770. PMID: 8160218.
Article4. Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, et al. North American Symptomatic Carotid Endarterectomy Trial Collaborators. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. N Engl J Med. 1998; 339:1415–1425. PMID: 9811916.
Article5. Rothwell PM, Gutnikov SA, Warlow CP. European Carotid Surgery Trialist's Collaboration. Reanalysis of the final results of the European Carotid Surgery Trial. Stroke. 2003; 34:514–523. PMID: 12574569.
Article6. Carr S, Farb A, Pearce WH, Virmani R, Yao JS. Atherosclerotic plaque rupture in symptomatic carotid artery stenosis. J Vasc Surg. 1996; 23:755–765. discussion 765-766. PMID: 8667496.
Article7. Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995; 92:657–671. PMID: 7634481.
Article8. Fisher M, Paganini-Hill A, Martin A, Cosgrove M, Toole JF, Barnett HJ, et al. Carotid plaque pathology: thrombosis, ulceration, and stroke pathogenesis. Stroke. 2005; 36:253–257. PMID: 15653581.
Article9. Redgrave JN, Lovett JK, Gallagher PJ, Rothwell PM. Histological assessment of 526 symptomatic carotid plaques in relation to the nature and timing of ischemic symptoms: the Oxford plaque study. Circulation. 2006; 113:2320–2328. PMID: 16651471.10. Kolodgie FD, Narula J, Haider N, Virmani R. Apoptosis in atherosclerosis. Does it contribute to plaque instability? Cardiol Clin. 2001; 19:127–139. ixPMID: 11787806.11. Shah PK. Mechanisms of plaque vulnerability and rupture. J Am Coll Cardiol. 2003; 41:15S–22S. PMID: 12644336.
Article12. Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994; 94:2493–2503. PMID: 7989608.
Article13. Loftus IM, Naylor AR, Bell PR, Thompson MM. Matrix metalloproteinases and atherosclerotic plaque instability. Br J Surg. 2002; 89:680–694. PMID: 12027977.
Article14. Aimes RT, Quigley JP. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem. 1995; 270:5872–5876. PMID: 7890717.15. Kähäri VM, Saarialho-Kere U. Matrix metalloproteinases in skin. Exp Dermatol. 1997; 6:199–213. PMID: 9450622.
Article16. Shah PK, Galis ZS. Matrix metalloproteinase hypothesis of plaque rupture: players keep piling up but questions remain. Circulation. 2001; 104:1878–1880. PMID: 11602486.17. Li Z, Li L, Zielke HR, Cheng L, Xiao R, Crow MT, et al. Increased expression of 72-kd type IV collagenase (MMP-2) in human aortic atherosclerotic lesions. Am J Pathol. 1996; 148:121–128. PMID: 8546199.18. Shah PK, Falk E, Badimon JJ, Fernandez-Ortiz A, Mailhac A, Villareal-Levy G, et al. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation. 1995; 92:1565–1569. PMID: 7664441.19. Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000; 20:1262–1275. PMID: 10807742.20. Stary HC. Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler Thromb Vasc Biol. 2000; 20:1177–1178. PMID: 10807728.21. Lovett JK, Gallagher PJ, Rothwell PM. Reproducibility of histological assessment of carotid plaque: implications for studies of carotid imaging. Cerebrovasc Dis. 2004; 18:117–123. PMID: 15218276.
Article22. Bassiouny HS, Davis H, Massawa N, Gewertz BL, Glagov S, Zarins CK. Critical carotid stenoses: morphologic and chemical similarity between symptomatic and asymptomatic plaques. J Vasc Surg. 1989; 9:202–212. PMID: 2537432.
Article23. Morgan AR, Rerkasem K, Gallagher PJ, Zhang B, Morris GE, Calder PC, et al. Differences in matrix metalloproteinase-1 and matrix metalloproteinase-12 transcript levels among carotid atherosclerotic plaques with different histopathological characteristics. Stroke. 2004; 35:1310–1315. PMID: 15073384.
Article24. European Carotid Plaque Study Group. Carotid artery plaque composition--relationship to clinical presentation and ultrasound B-mode imaging. Eur J Vasc Endovasc Surg. 1995; 10:23–30. PMID: 7633965.25. Jeziorska M, McCollum C, Woolley DE. Calcification in atherosclerotic plaque of human carotid arteries: associations with mast cells and macrophages. J Pathol. 1998; 185:10–17. PMID: 9713354.
Article26. Spagnoli LG, Mauriello A, Sangiorgi G, Fratoni S, Bonanno E, Schwartz RS, et al. Extracranial thrombotically active carotid plaque as a risk factor for ischemic stroke. JAMA. 2004; 292:1845–1852. PMID: 15494582.
Article27. Nikkari ST, O'Brien KD, Ferguson M, Hatsukami T, Welgus HG, Alpers CE, et al. Interstitial collagenase (MMP-1) expression in human carotid atherosclerosis. Circulation. 1995; 92:1393–1398. PMID: 7664418.
Article28. Sukhova GK, Schönbeck U, Rabkin E, Schoen FJ, Poole AR, Billinghurst RC, et al. Evidence for increased collagenolysis by interstitial collagenases-1 and -3 in vulnerable human atheromatous plaques. Circulation. 1999; 99:2503–2509. PMID: 10330380.
Article29. Loftus IM, Naylor AR, Goodall S, Crowther M, Jones L, Bell PR, et al. Increased matrix metalloproteinase-9 activity in unstable carotid plaques. A potential role in acute plaque disruption. Stroke. 2000; 31:40–47. PMID: 10625713.30. Alvarez B, Ruiz C, Chacón P, Alvarez-Sabin J, Matas M. Serum values of metalloproteinase-2 and metalloproteinase-9 as related to unstable plaque and inflammatory cells in patients with greater than 70% carotid artery stenosis. J Vasc Surg. 2004; 40:469–475. PMID: 15337875.
Article31. Sluijter JP, Pulskens WP, Schoneveld AH, Velema E, Strijder CF, Moll F, et al. Matrix metalloproteinase 2 is associated with stable and matrix metalloproteinases 8 and 9 with vulnerable carotid atherosclerotic lesions: a study in human endarterectomy specimen pointing to a role for different extracellular matrix metalloproteinase inducer glycosylation forms. Stroke. 2006; 37:235–239. PMID: 16339461.32. Molloy KJ, Thompson MM, Jones JL, Schwalbe EC, Bell PR, Naylor AR, et al. Unstable carotid plaques exhibit raised matrix metalloproteinase-8 activity. Circulation. 2004; 110:337–343. PMID: 15226217.
Article33. Turu MM, Krupinski J, Catena E, Rosell A, Montaner J, Rubio F, et al. Intraplaque MMP-8 levels are increased in asymptomatic patients with carotid plaque progression on ultrasound. Atherosclerosis. 2006; 187:161–169. PMID: 16259988.
Article34. Higashikata T, Yamagishi M, Higashi T, Nagata I, Iihara K, Miyamoto S, et al. Altered expression balance of matrix metalloproteinases and their inhibitors in human carotid plaque disruption: results of quantitative tissue analysis using real-time RT-PCR method. Atherosclerosis. 2006; 185:165–172. PMID: 16039658.
Article35. van Oostrom O, Velema E, Schoneveld AH, de Vries JP, de Bruin P, Seldenrijk CA, et al. Age-related changes in plaque composition: a study in patients suffering from carotid artery stenosis. Cardiovasc Pathol. 2005; 14:126–134. PMID: 15914297.36. Galis ZS, Kranzhöfer R, Fenton JW 2nd, Libby P. Thrombin promotes activation of matrix metalloproteinase-2 produced by cultured vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1997; 17:483–489. PMID: 9102166.
Article37. Kunte H, Amberger N, Busch MA, Rückert RI, Meiners S, Harms L. Markers of instability in high-risk carotid plaques are reduced by statins. J Vasc Surg. 2008; 47:513–522. PMID: 18295103.
Article38. Suzue A, Uno M, Kitazato KT, Nishi K, Yagi K, Liu H, et al. Comparison between early and late carotid endarterectomy for symptomatic carotid stenosis in relation to oxidized low-density lipoprotein and plaque vulnerability. J Vasc Surg. 2007; 46:870–875. PMID: 17980272.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnostic and Therapeutic Approach of Carotid and Cerebrovascular Plaque on the Basis of Vessel Imaging
- General principles of carotid Doppler ultrasonography
- Frequency of Combined Atherosclerotic Disease of the Coronary, Periphery, and Carotid Arteries Found by Angiography
- High-Resolusion Magnetic Resonance Imaging of Carotid Atherosclerotic Plaque
- Histological Evidence of Artery to Artery Embolism from Calcified Atherosclerotic Plaque of Carotid Artery