J Clin Neurol.
2008 Jun;4(2):67-74. 10.3988/jcn.2008.4.2.67.
Intracranial Hemodynamic Changes During Adult Moyamoya Disease Progression
- Affiliations
-
- 1Department of Neurology, Hospital and College of Medicine, Chungnam National University, Daejeon, Korea. jeikim@cnu.ac.kr
- 2Department of Information and Statistics, College of Natural Science, Chungnam National University, Daejeon, Korea.
- KMID: 2178925
- DOI: http://doi.org/10.3988/jcn.2008.4.2.67
Abstract
- Background and purpose
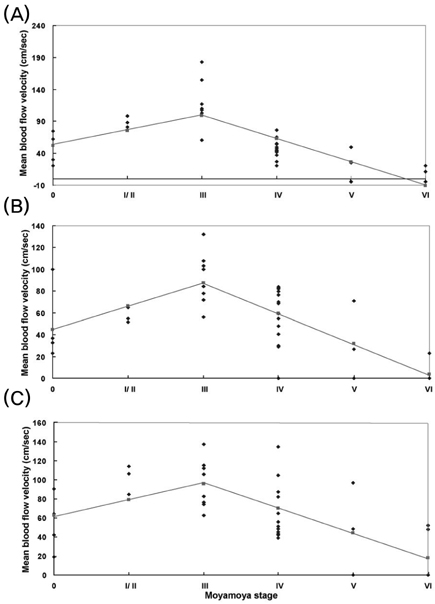
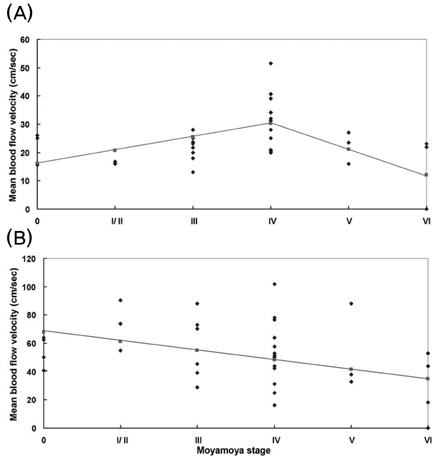
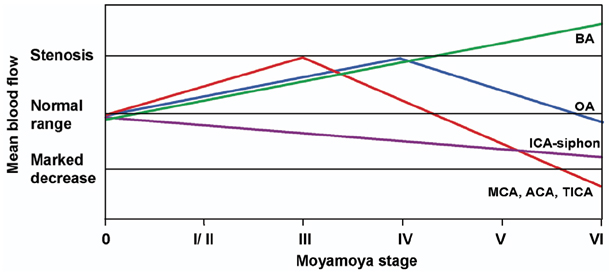
This study evaluated the changes in blood flow velocity in the anterior and posterior intracranial circulations according to the progression of moyamoya disease in adult patients. Methods: We evaluated Suzuki's angiographic stage and mean blood flow velocity (MBFV) changes in intracranial vessels from both sides in 19 adult moyamoya patients. We then analyzed the linearity of MBFV changes from early to late moyamoya stages in each intracranial vessel using piecewise linear regression models. Results: The MBFV in the middle cerebral artery, terminal internal carotid artery, and anterior cerebral artery increased non linearly until stage III, and then decreased progressively up to stage VI. The ophthalmic artery also showed nonlinear velocity changes, with an increase in MBFV up to stage IV, followed by a decrease in MBFV up to stage VI. The MBFV of the basilar artery increased linearly from a normal velocity at an early moyamoya stage to a stenotic velocity at a late stage. There was no statistically significant regression model for the relationship between the MBFV in the posterior cerebral artery and moyamoya stage. Conclusions: The nonlinear and/or linear MBFV changes associated with variable intracranial vessels might be useful in initial and follow-up evaluations of different stages of moyamoya disease.
MeSH Terms
Figure
Cited by 1 articles
-
Preliminary Study of Neurocognitive Dysfunction in Adult Moyamoya Disease and Improvement after Superficial Temporal Artery-Middle Cerebral Artery Bypass
Hyun Joo Baek, Seung Young Chung, Moon Sun Park, Seong Min Kim, Ki Suk Park, Hee Un Son
J Korean Neurosurg Soc. 2014;56(3):188-193. doi: 10.3340/jkns.2014.56.3.188.
Reference
-
1. Suzuki J, Takaku A. Cerebrovascular "moyamoya" disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969. 20:288–299.2. Takase K, Kashihara M, Hashimoto T. Transcranial Doppler ultrasonography in patients with moyamoya disease. Clin Neurol Neurosurg. 1997. 99:Suppl 2. S101–S105.
Article3. Lee YS, Jung KH, Roh JK. Diagnosis of moyamoya disease with transcranial Doppler sonography: correlation study with magnetic resonance angiography. J Neuroimaging. 2004. 14:319–323.
Article4. Yamauchi T, Tada M, Houkin K, Tanaka T, Nakamura Y, Kuroda S, et al. Linkage of familial moyamoya disease (spontaneous occlusion of the circle of Willis) to chromosome 17q25. Stroke. 2000. 31:930–935.
Article5. Suzuki J. Moyamoya Disease. 1983. Berlin: Springer-Verlag;143–185.6. Hoshi H, Ohnishi T, Jinnouchi S, Futami S, Nagamachi S, Kodama T, et al. Cerebral blood flow study in patients with moyamoya disease evaluated by IMP SPECT. J Nucl Med. 1994. 35:44–50.7. Miyamoto S, Kikuchi H, Karasawa J, Nagata I, Ikota T, Takeuchi S. Study of the posterior circulation in moyamoya disease. Clinical and neuroradiological evaluation. J Neurosurg. 1984. 61:1032–1037.8. Satoh S, Shibuya H, Matsushima Y, Suzuki S. Analysis of the angiographic findings in cases of childhood moyamoya disease. Neuroradiology. 1988. 30:111–119.
Article9. Kelly ME, Bell-Stephens TE, Marks MP, Do HM, Steinberg GK. Progression of unilateral moyamoya disease: A clinical series. Cerebrovasc Dis. 2006. 22:109–115.
Article10. Kuroda S, Ishikawa T, Houkin K, Nanba R, Hokari M, Iwasaki Y. Incidence and clinical features of disease progression in adult moyamoya disease. Stroke. 2005. 36:2148–2153.
Article11. Neter J, Wasserman W, Kunter MH. Applied Linear Statistical Models. 1990. 3rd ed. Homewood: Richard D. Irwin;370–373.12. Fischer AQ, Truemper EJ. Newell DW, Aaslid R, editors. Pediatric applications: II. Cerebrovascular control and application to specific disease processes. Transcranial Doppler. 1992. New York: Raven Press;245–268.13. Muttaqin Z, Ohba S, Arita K, Nakahara T, Pant B, Uozumi T, et al. Cerebral circulation in moyamoya disease: a clinical study using transcranial Doppler sonography. Surg Neurol. 1993. 40:306–313.
Article14. Fukui M. Current state of study on moyamoya disease in Japan. Surg Neurol. 1997. 47:138–143.
Article15. McAuley DJ, Poskitt K, Steinbok P. Predicting stroke risk in pediatric moyamoya disease with xenon-enhanced computed tomography. Neurosurgery. 2004. 55:327–332.
Article16. Fukui M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis ('moyamoya' disease). Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare, Japan. Clin Neurol Neurosurg. 1997. 99:Suppl 2. S238–S240.17. AbuRahma AF, Copeland SE. Bilateral internal carotid artery occlusion: natural history and surgical alternatives. Cardiovasc Surg. 1998. 6:579–583.
Article18. Catala M, Rancurel G, Raynaud C, Leder S, Kieffer E, Koskas F. Bilateral occlusion of the internal carotid arteries. Analysis of a series of 19 patients. Rev Neuro (Paris). 1995. 151:648–656.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Moyamoya-like Disease Associated with Intracranial Aneurysm
- Intracranial Aneurysm Associated with Moyamoya Disease
- A Case of Moyamoya Disease with Right Homonymous Hemianopsia
- The News on Moyamoya Disease: Review Article
- Anesthesia for a Patient with Moyamoya Disease presenting for Emergency Cesarean Section: A case report