J Gynecol Oncol.
2013 Oct;24(4):352-358. 10.3802/jgo.2013.24.4.352.
Improvements to the FIGO staging for ovarian cancer: reconsideration of lymphatic spread and intraoperative tumor rupture
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Seoul National University Bundang Hospital, Seongnam, Korea.
- 2Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Korea. kjwksh@snu.ac.kr
- 3Department of Obstetrics and Gynecology, Hanvit Women's Hospital, Ansan, Korea.
- 4Department of Obstetrics and Gynecology, SMG-SNU Boramae Medical Center, Seoul, Korea.
- 5Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 6WCU Biomodulation Major, Department of Agricultural Biotechnology, Seoul National University, Seoul, Korea.
- KMID: 2177877
- DOI: http://doi.org/10.3802/jgo.2013.24.4.352
Abstract
OBJECTIVE
To evaluate the improvement in prognosis prediction with reassignment of International Federation of Gynecology and Obstetrics (FIGO) stages for ovarian carcinoma.
METHODS
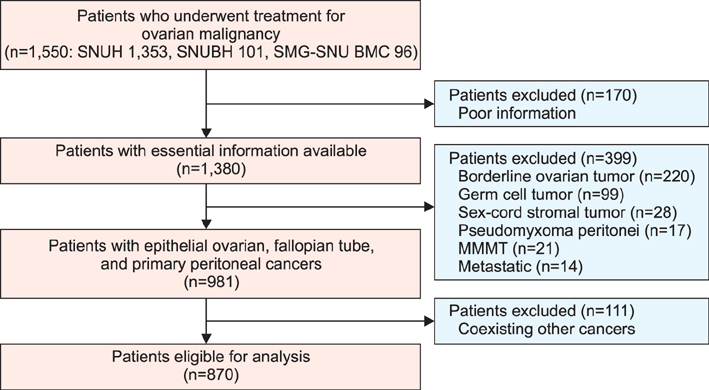
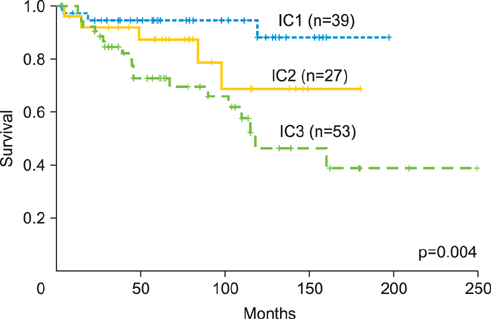
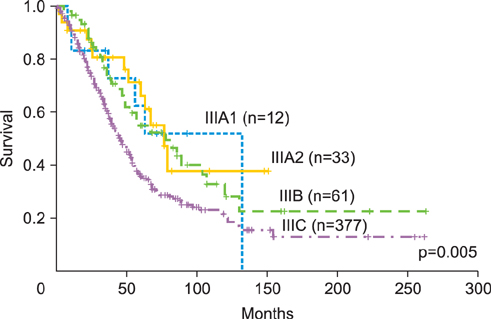
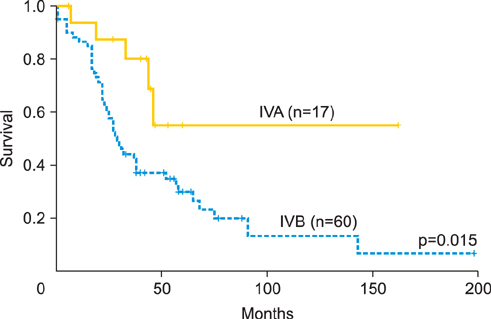
This was a retrospective study of patients with epithelial ovarian, fallopian tube, and primary peritoneal cancers. Sub-staging criteria used in stage reassignment were defined as follows: surgical spillage (IC1), capsule rupture before surgery or tumor on the surface (IC2), and positive cytology results (IC3); microscopic (IIB1) and macroscopic (IIB2) pelvic spread; microscopic extrapelvic spread (IIIA1) and retroperitoneal lymph node (LN) metastasis without extrapelvic spread (IIIA2); and supraclavicular LN metastasis (IVA) and other distant metastasis (IVB). Survival outcomes associated with the current and reassigned stages were compared.
RESULTS
Overall, 870 patients were eligible for analysis. The median follow-up period was 45 months (range, 0 to 263 months). The 5-year overall survival rates (5YSRs) according to the current staging were 93.5% (IA), 82.5% (IC), 75.0% (IIB), 74.5% (IIC), 57.5% (IIIA), 54.0% (IIIB), 38.5% (IIIC), and 33.0% (IV). The 5YSRs of patients with IC1, IC2, and IC3 after sub-staging were 92.0%, 85.0%, and 71.0%, respectively (p=0.004). Patients who were reassigned to stage IIIA2 had a better 5YSR than those with extrapelvic tumors >2 cm (66.3% vs. 35.8%; p=0.005). Additionally, patients with newly assigned stage IVA disease had a significantly better 5YSR than those with stage IVB disease (52.0% vs. 28.0%; p=0.015).
CONCLUSION
The modified FIGO staging for ovarian carcinoma appears superior to the current staging for discriminating survival outcomes of patients with surgical spillage, retroperitoneal LN metastasis without extrapelvic peritoneal involvement, or distant metastasis to supraclavicular LNs.
Keyword
MeSH Terms
Figure
Reference
-
1. Oh HK, Sin JI, Choi J, Park SH, Lee TS, Choi YS. Overexpression of CD73 in epithelial ovarian carcinoma is associated with better prognosis, lower stage, better differentiation and lower regulatory T cell infiltration. J Gynecol Oncol. 2012; 23:274–281.2. Kim HS, Ahn JH, Chung HH, Kim JW, Park NH, Song YS, et al. Impact of intraoperative rupture of the ovarian capsule on prognosis in patients with early-stage epithelial ovarian cancer: a meta-analysis. Eur J Surg Oncol. 2013; 39:279–289.3. Goudge CS, Li Z, Downs LS Jr. The influence of intraoperative tumor rupture on recurrence risk in Stage Ic epithelial ovarian cancer. Eur J Gynaecol Oncol. 2009; 30:25–28.4. Seidman JD, Cosin JA, Wang BG, Alsop S, Yemelyanova A, Fields A, et al. Upstaging pathologic stage I ovarian carcinoma based on dense adhesions is not warranted: a clinicopathologic study of 84 patients originally classified as FIGO stage II. Gynecol Oncol. 2010; 119:250–254.5. Berek JS. Lymph node-positive stage IIIC ovarian cancer: a separate entity? Int J Gynecol Cancer. 2009; 19:Suppl 2. S18–S20.6. Cliby WA, Aletti GD, Wilson TO, Podratz KC. Is it justified to classify patients to stage IIIC epithelial ovarian cancer based on nodal involvement only? Gynecol Oncol. 2006; 103:797–801.7. Baek SJ, Park JY, Kim DY, Kim JH, Kim YM, Kim YT, et al. Stage IIIC epithelial ovarian cancer classified solely by lymph node metastasis has a more favorable prognosis than other types of stage IIIC epithelial ovarian cancer. J Gynecol Oncol. 2008; 19:223–228.8. Denny L, Quinn M, Hacker N. FIGO cancer report 2012. Int J Gynaecol Obstet. 2012; 119:Suppl 2. S89.9. Vergote I, De Brabanter J, Fyles A, Bertelsen K, Einhorn N, Sevelda P, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet. 2001; 357:176–182.10. Kodama S, Kase H, Tanaka K, Matsui K. Multivariate analysis of prognostic factors in patients with endometrial cancer. Int J Gynaecol Obstet. 1996; 53:23–30.11. Bakkum-Gamez JN, Richardson DL, Seamon LG, Aletti GD, Powless CA, Keeney GL, et al. Influence of intraoperative capsule rupture on outcomes in stage I epithelial ovarian cancer. Obstet Gynecol. 2009; 113:11–17.12. Raungkaewmanee S, Tangjitgamol S, Manusirivithaya S, Srijaipracharoen S, Thavaramara T. Platelet to lymphocyte ratio as a prognostic factor for epithelial ovarian cancer. J Gynecol Oncol. 2012; 23:265–273.13. Bachmann C, Bachmann S, Fehm T, Staebler A, Becker S, Rothmund R, et al. Nodal status: its impact on prognosis in advanced ovarian cancer. J Cancer Res Clin Oncol. 2012; 138:261–267.14. Santillan A, Karam AK, Li AJ, Giuntoli R 2nd, Gardner GJ, Cass I, et al. Secondary cytoreductive surgery for isolated nodal recurrence in patients with epithelial ovarian cancer. Gynecol Oncol. 2007; 104:686–690.15. Benedetti Panici P, Perniola G, Angioli R, Zullo MA, Manci N, Palaia I, et al. Bulky lymph node resection in patients with recurrent epithelial ovarian cancer: impact of surgery. Int J Gynecol Cancer. 2007; 17:1245–1251.16. Powless CA, Aletti GD, Bakkum-Gamez JN, Cliby WA. Risk factors for lymph node metastasis in apparent early-stage epithelial ovarian cancer: implications for surgical staging. Gynecol Oncol. 2011; 122:536–540.17. Pickel H, Lahousen M, Stettner H, Girardi F. The spread of ovarian cancer. Baillieres Clin Obstet Gynaecol. 1989; 3:3–12.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognostic significance of tumor laterality in advanced ovarian cancer
- FIGO staging in ovarian carcinoma and histological subtypes
- Revised FIGO Staging System
- Revised International Federation of Gynecology and Obstetrics (FIGO) staging systems in gynecologic malignancies
- Comparison of single-port laparoscopy and laparotomy in early ovarian cancer surgical staging