J Breast Cancer.
2014 Dec;17(4):308-313. 10.4048/jbc.2014.17.4.308.
Meeting Highlights: The First Korean Breast Cancer Treatment Consensus Conference
- Affiliations
-
- 1Department of Surgery, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 2Department of Hematology-Oncology, Ehwa Womans University Hospital, Seoul, Korea.
- 3Department of Surgery, Kwandong University College of Medicine, Goyang, Korea.
- 4Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5Department of Surgery, Chungnam National University Hospital, Daejeon, Korea.
- 6Department of Surgery, Seoul National University Bundang Hospital, Seongnam, Korea.
- 7Department of Oncology, University of Ulsan College of Medicine, Seoul, Korea. khjung@amc.seoul.kr
- 8Department of Surgery, University of Ulsan College of Medicine, Seoul, Korea. brdrson@korea.com
- 9The Korean Breast Cancer Society, Seoul, Korea.
- KMID: 2176120
- DOI: http://doi.org/10.4048/jbc.2014.17.4.308
Abstract
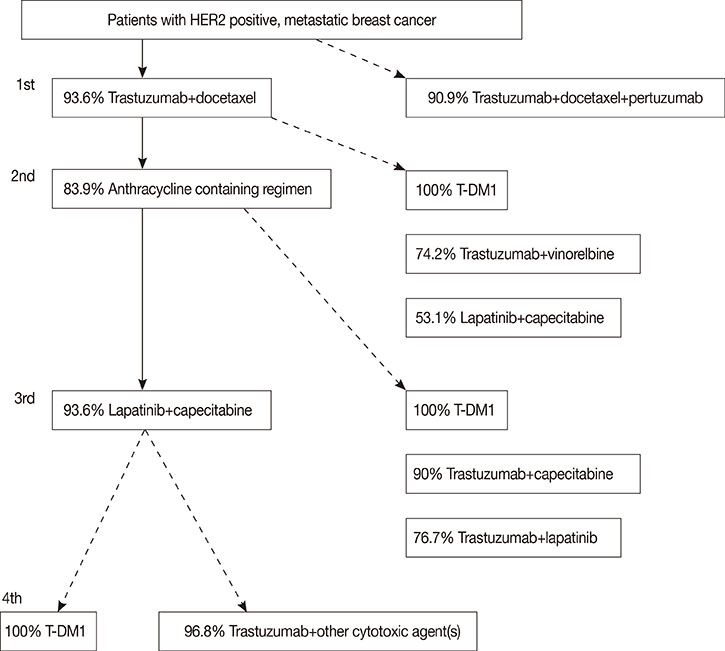
- The first Korean Breast Cancer Treatment Consensus Conference Expert Panel reviewed and endorsed new evidence on aspects of local and regional therapies and diagnostic procedures that support the conservative application of results from recent clinical trials. This conference clarified the barriers that limit the application of recent clinical trial results, such as questions about level of evidence, differences between the setting of clinical trials and that of daily clinical practice, and medical necessities and environment. Detailed decisions recommended for the treatment and diagnosis, according to the from the consensus conference, are recorded including details of the votes. These recommendations differed in the degree of support for clinical consideration of disease extent and host factors, medical necessities, and environment.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Meeting Highlights: The Second Consensus Conference for Breast Cancer Treatment in Korea
Seeyoun Lee, In Hae Park, Seho Park, Joohyuk Sohn, Joon Jeong, Sung Gwe Ahn, Ik Jae Lee, Hae Kyung Lee, Seung Ah Lee, Won Park, Kyung-Hun Lee, Sung-Won Kim, Sang-Ah Han, Kyung Hae Jung, Byung Ho Son
J Breast Cancer. 2017;20(3):228-233. doi: 10.4048/jbc.2017.20.3.228.
Reference
-
1. Ko BS, Noh WC, Kang SS, Park BW, Kang EY, Paik NS, et al. Changing patterns in the clinical characteristics of Korean breast cancer from 1996-2010 using an online nationwide breast cancer database. J Breast Cancer. 2012; 15:393–400.
Article2. Goldhirsch A, Wood WC, Senn HJ, Glick JH, Gelber RD. International Consensus Panel on the Treatment of Primary Breast Cancer. Fifth International Conference on Adjuvant Therapy of Breast Cancer, St. Gallen, March 1995. Eur J Cancer. 1995; 31:1754–1759.3. Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013; 24:2206–2223.
Article4. Glick JH. Meeting highlights: adjuvant therapy for breast cancer. J Natl Cancer Inst. 1988; 80:471–475.
Article5. McCulloch P, Taylor I, Sasako M, Lovett B, Griffin D. Randomised trials in surgery: problems and possible solutions. BMJ. 2002; 324:1448–1451.
Article6. de Boer M, van Deurzen CH, van Dijck JA, Borm GF, van Diest PJ, Adang EM, et al. Micrometastases or isolated tumor cells and the outcome of breast cancer. N Engl J Med. 2009; 361:653–663.
Article7. Giuliano AE, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010; 252:426–432.8. Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol. 2013; 14:297–305.
Article9. Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013; 310:1455–1461.
Article10. Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013; 14:609–618.
Article11. Hughes KS, Schnaper LA, Bellon JR, Cirrincione CT, Berry DA, McCormick B, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013; 31:2382–2387.
Article12. Kunkler I. PRIME II breast cancer trial. Clin Oncol (R Coll Radiol). 2004; 16:447–448.
Article13. Swain SM, Kim SB, Cortés J, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013; 14:461–471.
Article14. Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012; 367:1783–1791.
Article15. Cameron D, Casey M, Oliva C, Newstat B, Imwalle B, Geyer CE. Lapatinib plus capecitabine in women with HER-2-positive advanced breast cancer: final survival analysis of a phase III randomized trial. Oncologist. 2010; 15:924–934.
Article16. Burstein HJ, Keshaviah A, Baron AD, Hart RD, Lambert-Falls R, Marcom PK, et al. Trastuzumab plus vinorelbine or taxane chemotherapy for HER2-overexpressing metastatic breast cancer: the trastuzumab and vinorelbine or taxane study. Cancer. 2007; 110:965–972.
Article17. Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010; 375:377–384.
Article18. Untch M, Fasching PA, Konecny GE, Hasmüller S, Lebeau A, Kreienberg R, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol. 2011; 29:3351–3357.
Article19. Sabel MS. The need for axillary lymph node dissection in T1/T2 breast cancer surgery: counterpoint. Cancer Res. 2013; 73:7156–7160.
Article20. Barranger E, Houvenaeghel G, Classe JM. Axillary support in breast cancer: survey practice in France. Gynecol Obstet Fertil. 2013; 41:433–436.21. Kim Z, Min SY, Yoon CS, Lee HJ, Lee JS, Youn HJ, et al. The basic facts of Korean breast cancer in 2011: results of a nationwide survey and breast cancer registry database. J Breast Cancer. 2014; 17:99–106.
Article22. Colditz GA, Bohlke K, Berkey CS. Breast cancer risk accumulation starts early: prevention must also. Breast Cancer Res Treat. 2014; 145:567–579.
Article23. Shin HR, Joubert C, Boniol M, Hery C, Ahn SH, Won YJ, et al. Recent trends and patterns in breast cancer incidence among Eastern and Southeastern Asian women. Cancer Causes Control. 2010; 21:1777–1785.
Article24. Wu AH, Wan P, Hankin J, Tseng CC, Yu MC, Pike MC. Adolescent and adult soy intake and risk of breast cancer in Asian-Americans. Carcinogenesis. 2002; 23:1491–1496.
Article25. Wu AH, Yu MC, Tseng CC, Stanczyk FZ, Pike MC. Dietary patterns and breast cancer risk in Asian American women. Am J Clin Nutr. 2009; 89:1145–1154.
Article26. Ziegler RG, Hoover RN, Nomura AM, West DW, Wu AH, Pike MC, et al. Relative weight, weight change, height, and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1996; 88:650–660.
Article27. Ziegler RG, Hoover RN, Pike MC, Hildesheim A, Nomura AM, West DW, et al. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993; 85:1819–1827.
Article28. Clinical practice guidelines in oncology v.3. National Comprehensive Cancer Network;2014. Accessed August 25th, 2014. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Highlights of 10th St. Gallen Breast Cancer Conference: Systemic Adjuvant Treatment
- The Highligts of 28th Annual Meeting of San Antonio Breast Cancer Symposium
- HER-2/neu in Breast Cancer: Is Consensus Reached in Standard Testing?
- Meeting Highlights: The Second Consensus Conference for Breast Cancer Treatment in Korea
- National Guidelines for Breast Cancer Screening