J Breast Cancer.
2011 Dec;14(4):262-268. 10.4048/jbc.2011.14.4.262.
Clinical Significance of Annexin A1 Expression in Breast Cancer
- Affiliations
-
- 1Department of Surgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 2Department of Surgery and Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Pathology, KEPCO Medical Foundation, Hanil General Hospital, Seoul, Korea.
- 4Department of Surgery, Ewha Womans University School of Medicine, Seoul, Korea. mbit@ewha.ac.kr
- 5Department of Surgery, National Police Hospital, Seoul, Korea.
- KMID: 2175718
- DOI: http://doi.org/10.4048/jbc.2011.14.4.262
Abstract
- PURPOSE
The expression of Annexin A1 (ANXA1) is known to be reduced in human breast cancer; however, the role of ANXA1 expression in the development of breast cancer remains unclear. In this study, we determined the relationship between the expression features of ANXA1 and the prognostic factors of breast cancer.
METHODS
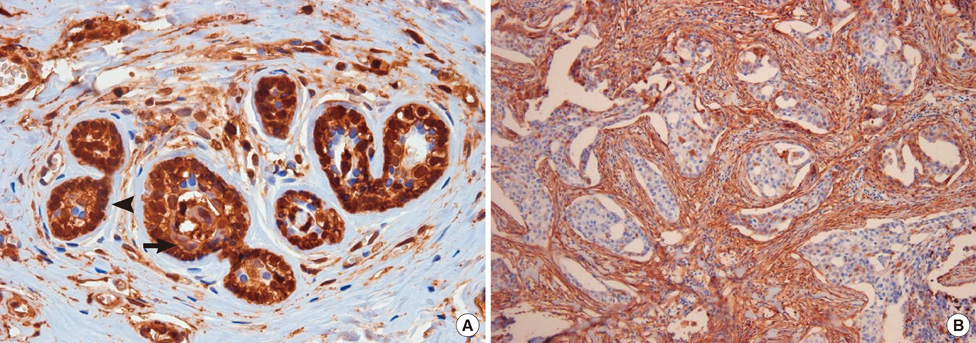
Human breast tissues were obtained from patients specimens who had undergone breast surgery or core needle biopsies. The patterns of ANXA1 expression were analyzed by immunohistochemical staining in relation to histopathological diagnosis, clinical characteristics and outcomes.
RESULTS
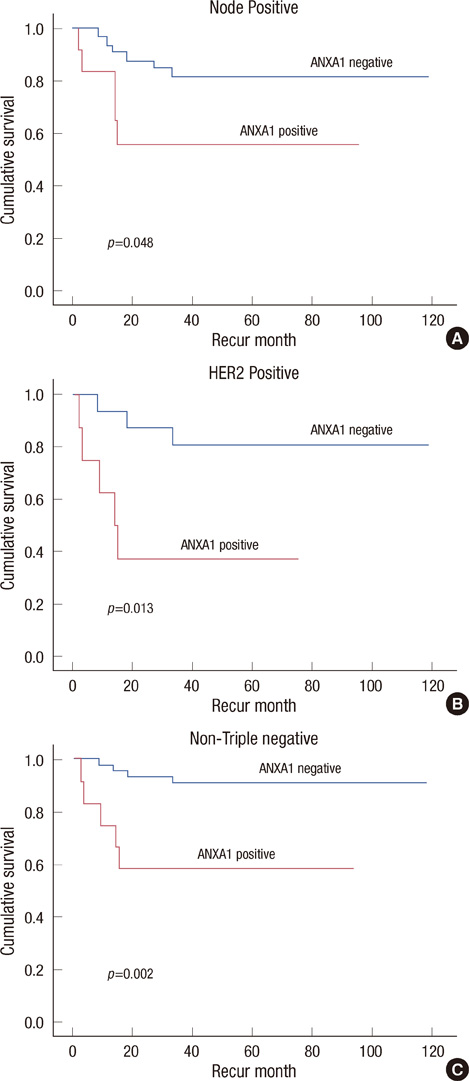
One hundred eighty-two cases were included and the mean age of the patients was 46.34 +/- 11.5 years. A significant loss of ANXA1 expression was noted in both ductal carcinoma in situ (DCIS) and invasive carcinomas compared to normal breast tissues (p<0.001) and benign breast diseases (p<0.001). There was a significant alteration in ANXA1 expression according to hormone receptor status (p<0.001), cancer intrinsic type (p<0.001), and nuclear grade (p=0.004) in invasive cancer. In a univariate analysis, ANXA1 positivity tended to be related with poor breast cancer-related survival (p=0.062); however, the same results was not realized in multivariate results (p=0.406). HER2 overexpression and TNM staging were significantly associated with relapse-free survivals (RFS) in the multivariate analysis (p=0.037, p=0.048, respectively). In particular, in node-positive patients (p=0.048), HER2 overexpressed patients (p=0.013), and non-triple negative breast cancer patients (p=0.002), ANXA1 overexpression was correlated with poor RFS.
CONCLUSION
Although significant loss of ANXA1 expression was noted in breast cancer including DCIS and invasive carcinoma, in cases of invasive cancer, overexpression of ANXA1 was related to unfavorable prognostic factors. And these results imply that ANXA1 plays dualistic roles and is involved in variable mechanisms related to cancer development and progression.
Keyword
MeSH Terms
Figure
Reference
-
1. Shen D, Nooraie F, Elshimali Y, Lonsberry V, He J, Bose S, et al. Decreased expression of annexin A1 is correlated with breast cancer development and progression as determined by a tissue microarray analysis. Hum Pathol. 2006. 37:1583–1591.
Article2. Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002. 82:331–371.
Article3. D'Acquisto F, Paschalidis N, Sampaio AL, Merghani A, Flower RJ, Perretti M. Impaired T cell activation and increased Th2 lineage commitment in Annexin-1-deficient T cells. Eur J Immunol. 2007. 37:3131–3142.4. Croxtall JD, Flower RJ. Lipocortin 1 mediates dexamethasone-induced growth arrest of the A549 lung adenocarcinoma cell line. Proc Natl Acad Sci U S A. 1992. 89:3571–3575.
Article5. Lozano JJ, Silberstein GB, Hwang S, Haindl AH, Rocha V. Developmental regulation of calcium-binding proteins (calelectrins and calpactin I) in mammary glands. J Cell Physiol. 1989. 138:503–510.
Article6. Horlick KR, Ganjianpour M, Frost SC, Nick HS. Annexin-I regulation in response to suckling and rat mammary cell differentiation. Endocrinology. 1991. 128:1574–1579.
Article7. Creutz CE, Kambouris NG, Snyder SL, Hamman HC, Nelson MR, Liu W, et al. Effects of the expression of mammalian annexins in yeast secretory mutants. J Cell Sci. 1992. 103(Pt 4):1177–1192.
Article8. Shen D, He J, Chang HR. In silico identification of breast cancer genes by combined multiple high throughput analyses. Int J Mol Med. 2005. 15:205–212.
Article9. Shen D, Chang HR, Chen Z, He J, Lonsberry V, Elshimali Y, et al. Loss of annexin A1 expression in human breast cancer detected by multiple high-throughput analyses. Biochem Biophys Res Commun. 2005. 326:218–227.
Article10. Zokas L, Glenney JR Jr. The calpactin light chain is tightly linked to the cytoskeletal form of calpactin I: studies using monoclonal antibodies to calpactin subunits. J Cell Biol. 1987. 105:2111–2121.
Article11. Glenney J, Zokas L. Antibodies to the N-terminus of calpactin II (p35) affect Ca2+ binding and phosphorylation by the epidermal growth factor receptor in vitro. Biochemistry. 1988. 27:2069–2076.
Article12. Wood GS, Warnke R. Suppression of endogenous avidin-binding activity in tissues and its relevance to biotin-avidin detection systems. J Histochem Cytochem. 1981. 29:1196–1204.
Article13. Man YG, Sang QX. The significance of focal myoepithelial cell layer disruptions in human breast tumor invasion: a paradigm shift from the "protease-centered" hypothesis. Exp Cell Res. 2004. 301:103–118.
Article14. Alldridge LC, Harris HJ, Plevin R, Hannon R, Bryant CE. The annexin protein lipocortin 1 regulates the MAPK/ERK pathway. J Biol Chem. 1999. 274:37620–37628.
Article15. Alldridge LC, Bryant CE. Annexin 1 regulates cell proliferation by disruption of cell morphology and inhibition of cyclin D1 expression through sustained activation of the ERK1/2 MAPK signal. Exp Cell Res. 2003. 290:93–107.
Article16. Bastian BC. Annexins in cancer and autoimmune diseases. Cell Mol Life Sci. 1997. 53:554–556.
Article17. Pencil SD, Toth M. Elevated levels of annexin I protein in vitro and in vivo in rat and human mammary adenocarcinoma. Clin Exp Metastasis. 1998. 16:113–121.18. Ahn SH, Sawada H, Ro JY, Nicolson GL. Differential expression of annexin I in human mammary ductal epithelial cells in normal and benign and malignant breast tissues. Clin Exp Metastasis. 1997. 15:151–156.19. Cicek M, Samant RS, Kinter M, Welch DR, Casey G. Identification of metastasis-associated proteins through protein analysis of metastatic MDA-MB-435 and metastasis-suppressed BRMS1 transfected-MDAMB-435 cells. Clin Exp Metastasis. 2004. 21:149–157.
Article20. Babbin BA, Lee WY, Parkos CA, Winfree LM, Akyildiz A, Perretti M, et al. Annexin I regulates SKCO-15 cell invasion by signaling through formyl peptide receptors. J Biol Chem. 2006. 281:19588–19599.
Article21. Wang Y, Serfass L, Roy MO, Wong J, Bonneau AM, Georges E. Annexin-I expression modulates drug resistance in tumor cells. Biochem Biophys Res Commun. 2004. 314:565–570.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The expression of annexin I and thymosin beta4 in cervical cancer
- The Expression Pattern of Annexin A1 in Urinary Bladder Urothelial Carcinoma and Its Clinicopathologic Significance
- The annexin II expression in invasive cervical cancer
- Role of Annexin A5 on Mitochondria-Dependent Apoptosis Induced by Tetramethoxystilbene in Human Breast Cancer Cells
- Efficacy of Annexin A1 Immunostaining in Bone Marrow for the Diagnosis of Hairy Cell Leukemia