J Gynecol Oncol.
2010 Dec;21(4):237-240. 10.3802/jgo.2010.21.4.237.
Outcomes of advanced and recurrent cervical cancer treated with cisplatin and generic topotecan: retrospective analysis in a tertiary care hospital in Thailand
- Affiliations
-
- 1Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Chiang Mai University Faculty of Medicine, Chiang Mai, Thailand. psuprase@mail.med.cmu.ac.th
- KMID: 2173547
- DOI: http://doi.org/10.3802/jgo.2010.21.4.237
Abstract
OBJECTIVE
Retrospective evaluation of the outcome of stage IVB, recurrent or persistent cervical cancer treated with cisplatin and generic topotecan (CT) in a tertiary care hospital in Thailand.
METHODS
The medical records of patients treated with CT regimen at Chiang Mai University Hospital between January 2005 and December 2007 were reviewed and analyzed. The treatment protocol consisted of IV topotecan 0.75 mg/m2 on days 1, 2, and 3; combined with cisplatin 50 mg/m2 IV on day 1 and repeated every 21 days until progression or unacceptable toxicity for a maximum of 6 cycles. The outcomes were evaluated based on the response rate, progression free survival (PFS), and overall survival (OS) by using the World Health Organization criteria. The adverse effects of the treatments were also determined.
RESULTS
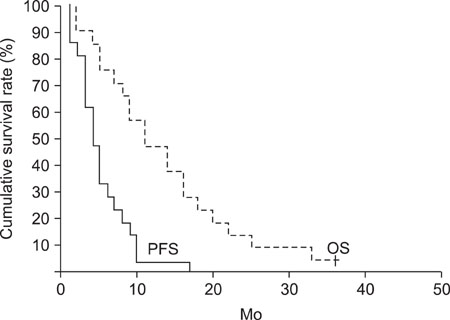
Twenty-one cervical cancer patients received the CT regimen. The tumor response rate was 28.6%. The median PFS and OS was 4 and 11 months, respectively. With 87 cycles of chemotherapy, the most common grade 3 & 4 hematologic toxicity was neutropenia (57.9%).
CONCLUSION
Advanced and recurrent cervical cancer patients treated with cisplatin and generic topotecan had a favorable outcome with manageable toxicity.
Keyword
MeSH Terms
Figure
Reference
-
1. Bonomi P, Blessing JA, Stehman FB, DiSaia PJ, Walton L, Major FJ. Randomized trial of three cisplatin dose schedules in squamous-cell carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 1985. 3:1079–1085.2. Long HJ 3rd, Bundy BN, Grendys EC Jr, Benda JA, McMeekin DS, Sorosky J, et al. Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: a Gynecologic Oncology Group Study. J Clin Oncol. 2005. 23:4626–4633.3. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981. 47:207–214.4. Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, ver. 3.0 [Internet]. 2006. cited 2010 Sep 20. Bethesda (MD): NIH; http://ctep.info.nih.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.5. Randall-Whitis LM, Monk BJ. Topotecan in the management of cervical cancer. Expert Opin Pharmacother. 2007. 8:227–236.6. Belozerov VE, Van Meir EG. Hypoxia inducible factor-1: a novel target for cancer therapy. Anticancer Drugs. 2005. 16:901–909.7. Fiorica JV, Blessing JA, Puneky LV, Secord AA, Hoffman JS, Yamada SD, et al. A Phase II evaluation of weekly topotecan as a single agent second line therapy in persistent or recurrent carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 2009. 115:285–289.8. Monk BJ, Sill MW, McMeekin DS, Cohn DE, Ramondetta LM, Boardman CH, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2009. 27:4649–4655.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of advanced ovarian cancer which was treated with topotecan after taxol-cisplatin treatment failed
- The role of topotecan as second-line chemotherapy in patients with recurrent epithelial ovarian cancer
- Effect of Topotecan in Combication with Other Antitumor Drugs in Vitro
- Study on Measure of Chemosensitivities to Topotecan, Cisplatin and Taxol Theraphy in Ovarian Cancer Cell Lines: Relationship with p53 and bcl-2 Expression and Apoptosis
- Long-term survival after intraperitoneal chemotherapy with paclitaxel-cisplatin for recurrent primary peritoneal cancer resistant to multiple lines of intravenous chemotherapy